This is a preprint.
Molecular and Serologic Diagnostic Technologies for SARS-CoV-2
- PMID: 35547240
- PMCID: PMC9094103
Molecular and Serologic Diagnostic Technologies for SARS-CoV-2
Abstract
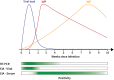
The COVID-19 pandemic has presented many challenges that have spurred biotechnological research to address specific problems. Diagnostics is one area where biotechnology has been critical. Diagnostic tests play a vital role in managing a viral threat by facilitating the detection of infected and/or recovered individuals. From the perspective of what information is provided, these tests fall into two major categories, molecular and serological. Molecular diagnostic techniques assay whether a virus is present in a biological sample, thus making it possible to identify individuals who are currently infected. Additionally, when the immune system is exposed to a virus, it responds by producing antibodies specific to the virus. Serological tests make it possible to identify individuals who have mounted an immune response to a virus of interest and therefore facilitate the identification of individuals who have previously encountered the virus. These two categories of tests provide different perspectives valuable to understanding the spread of SARS-CoV-2. Within these categories, different biotechnological approaches offer specific advantages and disadvantages. Here we review the categories of tests developed for the detection of the SARS-CoV-2 virus or antibodies against SARS-CoV-2 and discuss the role of diagnostics in the COVID-19 pandemic.
Conflict of interest statement
Competing Interests AuthorCompeting InterestsLast ReviewedHalie M. RandoNone2021-01-20Christian BruefferEmployee and shareholder of SAGA Diagnostics AB.2020-11-11Ronan LordanNone2020-11-03Anna Ada DattoliNone2020-03-26David ManheimNone2022-03-15Jesse G. MeyerNone2022-01-06Ariel I. MundoNone2021-12-19Dimitri PerrinNone2020-11-11David MaiNone2021-01-08Nils WellhausenNone2020-11-03COVID-19 Review ConsortiumNone2021-01-16Anthony GitterFiled a patent application with the Wisconsin Alumni Research Foundation related to classifying activated T cells2020-11-10Casey S. GreeneNone2021-01-20
Figures
References
-
- Pathogenesis, Symptomatology, and Transmission of SARS-CoV-2 through Analysis of Viral Genomics and Structure Rando Halie M, MacLean Adam L, Lee Alexandra J, Lordan Ronan, Ray Sandipan, Bansal Vikas, Skelly Ashwin N, Sell Elizabeth, Dziak John J, Shinholster Lamonica, … Greene Casey S mSystems (2021-October-26) https://pubmed.ncbi.nlm.nih.gov/34698547/ DOI: 10.1128/msystems.00095-21 - DOI - PMC - PubMed
-
- Aggressively find, test, trace and isolate to beat COVID-19 Matukas Larissa M, Dhalla Irfan A, Laupacis Andreas Canadian Medical Association Journal (2020-September-09) https://doi.org/gh2jvk DOI: 10.1503/cmaj.202120 - DOI - PMC - PubMed
-
- COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study Jefferies Sarah, French Nigel, Gilkison Charlotte, Graham Giles, Hope Virginia, Marshall Jonathan, McElnay Caroline, McNeill Andrea, Muellner Petra, Paine Shevaun, … Priest Patricia The Lancet Public Health (2020-November) https://doi.org/ftzx DOI: 10.1016/s2468-2667(20)30225-5 - DOI - PMC - PubMed
-
- Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic Summers Jennifer, Cheng Hao-Yuan, Lin Hsien-Ho, Barnard Lucy Telfar, Kvalsvig Amanda, Wilson Nick, Baker Michael G The Lancet Regional Health - Western Pacific (2020-November) https://doi.org/ghrzb4 DOI: 10.1016/j.lanwpc.2020.100044 - DOI - PMC - PubMed
-
- Novel Coronavirus - China. https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON233 .
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
