Identification of Neoantigens and Construction of Immune Subtypes in Prostate Adenocarcinoma
- PMID: 35547260
- PMCID: PMC9081437
- DOI: 10.3389/fgene.2022.886983
Identification of Neoantigens and Construction of Immune Subtypes in Prostate Adenocarcinoma
Abstract
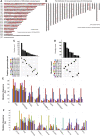
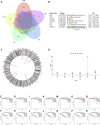
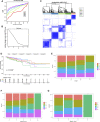
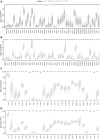
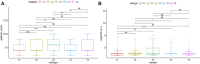
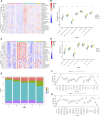
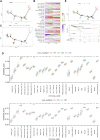
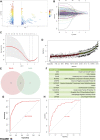
Background: Messenger ribonucleic acid (mRNA) vaccine has been considered as a potential therapeutic strategy and the next research hotspot, but their efficacy against prostate adenocarcinoma (PRAD) remains undefined. This study aimed to find potential antigens of PRAD for mRNA vaccine development and identify suitable patients for vaccination through immunophenotyping. Methods: Gene expression profiles and clinical information were obtained from TCGA and ICGC. GEPIA2 was used to calculate the prognostic index of the selected antigens. The genetic alterations were compared on cBioPortal and the correlation between potential antigen and immune infiltrating cells was explored by TIMER. ConsensusClusterPlus was used to construct a consistency matrix, and identify the immune subtypes. Graph learning-based dimensional reduction was performed to depict immune landscape. Boruta algorithm and LASSO logistic analysis were used to screen PRAD patients who may benefit from mRNA vaccine. Results: Seven potential tumor antigens selected were significantly positively associated with poor prognosis and the antigen-presenting immune cells (APCs) in PRAD, including ADA, FYN, HDC, NFKBIZ, RASSF4, SLC6A3, and UPP1. Five immune subtypes of PRAD were identified by differential molecular, cellular, and clinical characteristics in both cohorts. C3 and C5 had immune "hot" and immunosuppressive phenotype, On the contrary, C1&C2 had immune "cold" phenotype. Finally, the immune landscape characterization showed the immune heterogeneity among patients with PRAD. Conclusions: ADA, FYN, HDC, NFKBIZ, RASSF4, SLC6A3, and UPP1 are potential antigens for mRNA vaccine development against PRAD, and patients in type C1 and C2 are suitable for vaccination.
Keywords: immune landscape; immunotype; mRNA vaccine; prostate adenocarcinoma; tumor immune microenvironment.
Copyright © 2022 Gao, Wang, Chen, Zhang, Gao, Shang and Niu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Identification of tumor antigens and immune subtypes of pancreatic adenocarcinoma for mRNA vaccine development.Mol Cancer. 2021 Mar 1;20(1):44. doi: 10.1186/s12943-021-01310-0. Mol Cancer. 2021. PMID: 33648511 Free PMC article.
-
Screening of tumor antigens and immunogenic cell death landscapes of prostate adenocarcinoma for exploration of mRNA vaccine.Expert Rev Vaccines. 2024 Jan-Dec;23(1):830-844. doi: 10.1080/14760584.2024.2396086. Epub 2024 Sep 2. Expert Rev Vaccines. 2024. PMID: 39193620
-
Tumor-antigens and immune landscapes identification for prostate adenocarcinoma mRNA vaccine.Mol Cancer. 2021 Dec 6;20(1):160. doi: 10.1186/s12943-021-01452-1. Mol Cancer. 2021. PMID: 34872584 Free PMC article.
-
Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development.Mol Cancer. 2021 Mar 8;20(1):50. doi: 10.1186/s12943-021-01342-6. Mol Cancer. 2021. PMID: 33685460 Free PMC article.
-
Bioinformatics analyses for the identification of tumor antigens and immune subtypes of gastric adenocarcinoma.Front Genet. 2022 Dec 12;13:1068112. doi: 10.3389/fgene.2022.1068112. eCollection 2022. Front Genet. 2022. PMID: 36579327 Free PMC article.
References
-
- Boorjian S. A., Thompson R. H., Siddiqui S., Bagniewski S., Bergstralh E. J., Karnes R. J., et al. (2007). Long-term Outcome after Radical Prostatectomy for Patients with Lymph Node Positive Prostate Cancer in the Prostate Specific Antigen Era. J. Urol. 178 (3 Pt 1), 864–871. 10.1016/j.juro.2007.05.048 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

