Electron paramagnetic resonance spectroscopic studies of the electron transfer reaction of Hantzsch ester and a pyrylium salt
- PMID: 35547277
- PMCID: PMC9085297
- DOI: 10.1039/c8ra05693e
Electron paramagnetic resonance spectroscopic studies of the electron transfer reaction of Hantzsch ester and a pyrylium salt
Abstract
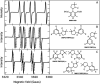
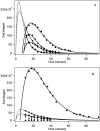
The oxidation of Hantzsch ester by a pyrylium cation takes place via electron-proton-electron transfer. The reaction was investigated with EPR spectroscopy using TEMPO and DMPO for inhibition and spin trapping, respectively, of the radicals appearing during the reaction. The present in-depth EPR study of the radical reactions of a NADH analogue indicate a complex electron transfer mechanism in the title reaction.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Oxidation of spin trap 5,5-dimethyl-1-pyrroline-1-oxide in an electron paramagnetic resonance study of the reaction of methemoglobin with hydrogen peroxide.Free Radic Biol Med. 1994 Apr;16(4):493-500. doi: 10.1016/0891-5849(94)90127-9. Free Radic Biol Med. 1994. PMID: 8005534
-
Spin-trapping studies of peroxynitrite decomposition and of 3-morpholinosydnonimine N-ethylcarbamide autooxidation: direct evidence for metal-independent formation of free radical intermediates.Arch Biochem Biophys. 1994 Apr;310(1):118-25. doi: 10.1006/abbi.1994.1147. Arch Biochem Biophys. 1994. PMID: 8161194
-
In Vivo and In Situ Detection of Macromolecular Free Radicals Using Immuno-Spin Trapping and Molecular Magnetic Resonance Imaging.Antioxid Redox Signal. 2018 May 20;28(15):1404-1415. doi: 10.1089/ars.2017.7390. Epub 2017 Dec 11. Antioxid Redox Signal. 2018. PMID: 29084431 Review.
-
Electron transfer between a tyrosyl radical and a cysteine residue in hemoproteins: spin trapping analysis.J Am Chem Soc. 2007 Nov 7;129(44):13493-501. doi: 10.1021/ja073349w. Epub 2007 Oct 16. J Am Chem Soc. 2007. PMID: 17939657
-
Detection and characterisation of radicals in biological materials using EPR methodology.Biochim Biophys Acta. 2014 Feb;1840(2):708-21. doi: 10.1016/j.bbagen.2013.03.034. Epub 2013 Apr 6. Biochim Biophys Acta. 2014. PMID: 23567797 Review.
Cited by
-
Flavylium Salts: A Blooming Core for Bioinspired Ionic Liquid Crystals.Chemistry. 2019 Oct 8;25(56):12966-12980. doi: 10.1002/chem.201901975. Epub 2019 Sep 18. Chemistry. 2019. PMID: 31418972 Free PMC article.
References
-
- Zhu X.-Q. Liu Y.-C. Cheng J.-P. J. Org. Chem. 1999;64:8980–8981. doi: 10.1021/jo9905571. - DOI
-
- Zhao B. Zhu X. Lu Y. Xia C.-Z. Cheng J.-P. Tetrahedron Lett. 2000;41:257–260. doi: 10.1016/S0040-4039(99)01762-1. - DOI
-
- Mauzerall D. Westheimer F. H. J. Am. Chem. Soc. 1955;77:2261–2264. doi: 10.1021/ja01613a070. - DOI
LinkOut - more resources
Full Text Sources

