Monocarboxylate Transporter 1 May Benefit Cerebral Ischemia via Facilitating Lactate Transport From Glial Cells to Neurons
- PMID: 35547368
- PMCID: PMC9081727
- DOI: 10.3389/fneur.2022.781063
Monocarboxylate Transporter 1 May Benefit Cerebral Ischemia via Facilitating Lactate Transport From Glial Cells to Neurons
Abstract
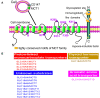
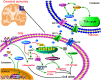
Monocarboxylate transporter 1 (MCT1) is expressed in glial cells and some populations of neurons. MCT1 facilitates astrocytes or oligodendrocytes (OLs) in the energy supplement of neurons, which is crucial for maintaining the neuronal activity and axonal function. It is suggested that MCT1 upregulation in cerebral ischemia is protective to ischemia/reperfusion (I/R) injury. Otherwise, its underlying mechanism has not been clearly discussed. In this review, it provides a novel insight that MCT1 may protect brain from I/R injury via facilitating lactate transport from glial cells (such as, astrocytes and OLs) to neurons. It extensively discusses (1) the structure and localization of MCT1; (2) the regulation of MCT1 in lactate transport among astrocytes, OLs, and neurons; and (3) the regulation of MCT1 in the cellular response of lactate accumulation under ischemic attack. At last, this review concludes that MCT1, in cerebral ischemia, may improve lactate transport from glial cells to neurons, which subsequently alleviates cellular damage induced by lactate accumulation (mostly in glial cells), and meets the energy metabolism of neurons.
Keywords: astrocytes; cerebral ischemia; lactate transport; monocarboxylate transporter 1 (MCT1); oligodendrocytes (OLs).
Copyright © 2022 Zhang, Wang, Bai, Dai and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

