Electrolyte and pH-sensitive amphiphilic alginate: synthesis, self-assembly and controlled release of acetamiprid
- PMID: 35547515
- PMCID: PMC9086226
- DOI: 10.1039/c8ra05503c
Electrolyte and pH-sensitive amphiphilic alginate: synthesis, self-assembly and controlled release of acetamiprid
Abstract
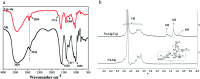
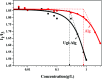
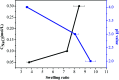
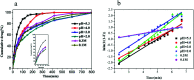
In this study, a pH-responsive amphiphilic alginate (Ugi-Alg) was synthesized via Ugi reaction without using a catalyst. The structure of Ugi-Alg was confirmed by FT-IR and 1H NMR spectroscopy. Amphiphilic alginate can form micelles in an aqueous medium due to it's amphiphilic nature.. The impacts of Na+ concentration and pH on the micelle size were characterized by dynamic light scattering (DLS) and transmission electron microscopy (TEM). The dynamic light scattering observations showed that micelle size increases with the decrease in Na+ concentration in aqueous solution. However, the micelle size decreases first and then increases as the pH value decreases from 5.3 to 2.0. Transmission electron microscopy confirmed that the mean size of micelles is 30-200 nm. In addition, a model hydrophobic pesticide (acetamiprid) was loaded in the micelles. The encapsulation efficiency and release behavior of micelles were studied, which could be controlled by Na+ concentration and pH. The results indicated that encapsulation efficiency of acetamiprid increases from 55% to 96% due to the increase in Na+ concentration from 0.01 M to 0.3 M. Moreover, with the decrease in pH from 5.3 to 2.0, encapsulation efficiency increases from 55% to 80%. Furthermore, the data of acetamiprid release kinetics could be well-fitted by the Weibull model.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


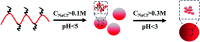

References
-
- Sharma S. Singh S. Ganguli A. K. Shanmugam V. Carbon. 2017;115:781–790. doi: 10.1016/j.carbon.2017.01.075. - DOI
LinkOut - more resources
Full Text Sources

