A biosensing system using a multiparameter nonlinear dynamic analysis of cardiomyocyte beating for drug-induced arrhythmia recognition
- PMID: 35547605
- PMCID: PMC9081091
- DOI: 10.1038/s41378-022-00383-1
A biosensing system using a multiparameter nonlinear dynamic analysis of cardiomyocyte beating for drug-induced arrhythmia recognition
Abstract
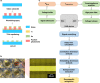
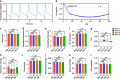
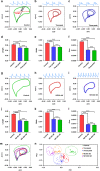
Cardiovascular disease is the number one cause of death in humans. Therefore, cardiotoxicity is one of the most important adverse effects assessed by arrhythmia recognition in drug development. Recently, cell-based techniques developed for arrhythmia recognition primarily employ linear methods such as time-domain analysis that detect and compare individual waveforms and thus fall short in some applications that require automated and efficient arrhythmia recognition from large datasets. We carried out the first report to develop a biosensing system that integrated impedance measurement and multiparameter nonlinear dynamic algorithm (MNDA) analysis for drug-induced arrhythmia recognition and classification. The biosensing system cultured cardiomyocytes as physiologically relevant models, used interdigitated electrodes to detect the mechanical beating of the cardiomyocytes, and employed MNDA analysis to recognize drug-induced arrhythmia from the cardiomyocyte beating recording. The best performing MNDA parameter, approximate entropy, enabled the system to recognize the appearance of sertindole- and norepinephrine-induced arrhythmia in the recording. The MNDA reconstruction in phase space enabled the system to classify the different arrhythmias and quantify the severity of arrhythmia. This new biosensing system utilizing MNDA provides a promising and alternative method for drug-induced arrhythmia recognition and classification in cardiological and pharmaceutical applications.
Keywords: Electrical and electronic engineering; Micro-optics.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures



Similar articles
-
Recognition of high-specificity hERG K+ channel inhibitor-induced arrhythmia in cardiomyocytes by automated template matching.Microsyst Nanoeng. 2021 Mar 16;7:24. doi: 10.1038/s41378-021-00251-4. eCollection 2021. Microsyst Nanoeng. 2021. PMID: 34567738 Free PMC article.
-
A biosensing system employing nonlinear dynamic analysis-assisted neural network for drug-induced cardiotoxicity assessment.Biosens Bioelectron. 2023 Feb 15;222:114923. doi: 10.1016/j.bios.2022.114923. Epub 2022 Nov 17. Biosens Bioelectron. 2023. PMID: 36455375
-
High-throughput rhythmic regulation of cardiomyocytes by integrated electrical stimulation and video-based automated analysis biosensing platform.Biosens Bioelectron. 2022 Aug 1;209:114252. doi: 10.1016/j.bios.2022.114252. Epub 2022 Apr 6. Biosens Bioelectron. 2022. PMID: 35405502
-
Human stem cell-derived cardiomyocytes in cellular impedance assays: bringing cardiotoxicity screening to the front line.Cardiovasc Toxicol. 2015 Apr;15(2):127-39. doi: 10.1007/s12012-014-9268-9. Cardiovasc Toxicol. 2015. PMID: 25134468 Review.
-
Anticancer drugs and cardiotoxicity: the role of cardiomyocyte and non-cardiomyocyte cells.Front Cardiovasc Med. 2024 Jul 11;11:1372817. doi: 10.3389/fcvm.2024.1372817. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39081368 Free PMC article. Review.
References
-
- Ebrahimi Z, Loni M, Daneshtalab M, Gharehbaghi A. A review on deep learning methods for ECG arrhythmia classification. Expert. Syst. Appl.: X. 2020;7:100033.
-
- Wang HD, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
