Development of a visible light, cross-linked GelMA hydrogel containing decellularized human amniotic particles as a soft tissue replacement for oral mucosa repair
- PMID: 35547651
- PMCID: PMC9087906
- DOI: 10.1039/c9ra03009c
Development of a visible light, cross-linked GelMA hydrogel containing decellularized human amniotic particles as a soft tissue replacement for oral mucosa repair
Abstract
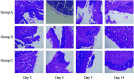
Early effective treatment of oral mucosal defects is the key to ensuring defect healing and functional recovery. The application of human amniotic membrane (HAM) in promoting wound healing has been shown to be safe and effective. However, amniotic membrane is thin, easy to tear and difficult to handle. Combined with the natural forces at play in the oral cavity, this has restricted the clinical applications of HAM for healing of mucosal defects. Methacrylated gelatin (GelMA) has good mechanical strength and adhesion, and can be used as a bionic repair film to attach to the damaged surface of oral mucosa, but GelMA lacks bioactive substances and cannot promote the rapid repair of oral mucosal defects. The aim of this study was to design a type of composite GelMA hydrogel mixed with decellularized human amniotic particles (dHAP) as an oral mucosa substitute, to promote regeneration of defective mucosa by stimulating rapid angiogenesis. The composite substitute GelMA-dHAP was easy to synthesize and store, and easy to operate for repair of oral mucosal defects. We show the angiogenic potential of GelMA-dHAP on chick chorioallontoic membrane and the curative effect of GelMA-dHAP as a treatment in the rabbit oral mucosa defect model. In conclusion, this study confirms the effectiveness of GelMA-dHAP as an ideal soft tissue substitute for the repair of oral mucosal defects, overcoming the shortcomings of using HAM or GelMA alone.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declared that there was no conflict of interest in the financial and publication of the work. All the authors gave their seal of approval to the manuscript.
Figures



References
-
- Piraino F. Selimovic S. Mol. Cell. Ther. 2015;2015:1–10.
-
- Rigal-Satourne J. C. Legeais J. M. Texier J. M. Savoldelli M. Renard J. P. Maurin J. F. Renard G. Invest. Ophthalmol. Visual Sci. 2000;4:S455.
LinkOut - more resources
Full Text Sources
Other Literature Sources

