Combinatorial Herpes Simplex Vaccine Strategies: From Bedside to Bench and Back
- PMID: 35547736
- PMCID: PMC9082490
- DOI: 10.3389/fimmu.2022.849515
Combinatorial Herpes Simplex Vaccine Strategies: From Bedside to Bench and Back
Abstract
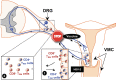
The development of vaccines against herpes simplex virus type 1 and type 2 (HSV1 and HSV-2) is an important goal for global health. In this review we reexamined (i) the status of ocular herpes vaccines in clinical trials; and (ii) discusses the recent scientific advances in the understanding of differential immune response between HSV infected asymptomatic and symptomatic individuals that form the basis for the new combinatorial vaccine strategies targeting HSV; and (iii) shed light on our novel "asymptomatic" herpes approach based on protective immune mechanisms in seropositive asymptomatic individuals who are "naturally" protected from recurrent herpetic diseases. We previously reported that phenotypically and functionally distinct HSV-specific memory CD8+ T cell subsets in asymptomatic and symptomatic HSV-infected individuals. Moreover, a better protection induced following a prime/pull vaccine approach that consists of first priming anti-viral effector memory T cells systemically and then pulling them to the sites of virus reactivation (e.g., sensory ganglia) and replication (e.g., eyes and vaginal mucosa), following mucosal administration of vectors expressing T cell-attracting chemokines. In addition, we reported that a combination of prime/pull vaccine approach with approaches to reverse T cell exhaustion led to even better protection against herpes infection and disease. Blocking PD-1, LAG-3, TIGIT and/or TIM-3 immune checkpoint pathways helped in restoring the function of antiviral HSV-specific CD8+ T cells in latently infected ganglia and increased efficacy and longevity of the prime/pull herpes vaccine. We discussed that a prime/pull vaccine strategy that use of asymptomatic epitopes, combined with immune checkpoint blockade would prove to be a successful herpes vaccine approach.
Keywords: asymptomatic; clinical trials; herpes simplex virus; immune checkpoint blockade; vaccines.
Copyright © 2022 Chentoufi, Dhanushkodi, Srivastava, Prakash, Coulon, Zayou, Vahed, Chentoufi, Hormi-Carver and BenMohamed.
Conflict of interest statement
Author HV was employed by TechImmune, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- McQuillan G, Kruszon-Moran D, Flagg EW, Paulose-Ram R. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14-49: United States, 2015-2016. NCHS Data Brief (2018) (304):1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

