Brief Research Report: Virus-Specific Humoral Immunity at Admission Predicts the Development of Respiratory Failure in Unvaccinated SARS-CoV-2 Patients
- PMID: 35547738
- PMCID: PMC9082065
- DOI: 10.3389/fimmu.2022.878812
Brief Research Report: Virus-Specific Humoral Immunity at Admission Predicts the Development of Respiratory Failure in Unvaccinated SARS-CoV-2 Patients
Erratum in
-
Corrigendum: Brief Research Report: Virus-Specific Humoral Immunity at Admission Predicts the Development of Respiratory Failure in Unvaccinated SARS-CoV-2 Patients.Front Immunol. 2022 Jun 30;13:965809. doi: 10.3389/fimmu.2022.965809. eCollection 2022. Front Immunol. 2022. PMID: 35990649 Free PMC article.
Abstract
Introduction: There is robust evidence indicating that the SARS-CoV-2-specific humoral response is associated with protection against severe disease. However, relatively little data exist regarding how the humoral immune response at the time of hospital admission correlates with disease severity in unimmunized patients. Our goal was toidentify variables of the humoral response that could potentially serve as prognostic markers for COVID-19 progressionin unvaccinated SARS-CoV-2 patients.
Methods: A prospective cross-sectional study was carried out in a cohort of 160 unimmunized, adult COVID-19 patients from the Hospital Universitario 12Octubre. Participants were classified into four clinical groups based on disease severity: non-survivors with respiratory failure (RF), RF survivors, patients requiring oxygen therapy and those not receiving oxygen therapy. Serum samples were taken on admission and IgM, IgG, IgG subclass antibody titers were determined by ELISA, and neutralizing antibody titersusing a surrogate neutralization assay. The differences in the antibody titers between groups and the association between the clinical and analytical characteristics of the patients and the antibody titers were analyzed.
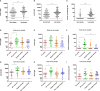
Results: Patients that developed RF and survived had IgM titers that were 2-fold higher than non-survivors (p = 0.001), higher levels of total IgG than those who developed RF and succumbed to infection (p< 0.001), and than patients who required oxygen therapy (p< 0.05), and had 5-fold higher IgG1 titers than RF non-survivors (p< 0.001) and those who needed oxygen therapy (p< 0.001), and 2-fold higher than patients that did not require oxygen therapy during admission (p< 0.05). In contrast, RF non-survivorshad the lowest neutralizing antibodylevels, which were significantly lower compared those with RF that survived (p = 0.03). A positive correlation was found between IgM, total IgG, IgG1 and IgG3 titers and neutralizing antibody titers in the total cohort (p ≤ 0.0036).
Conclusions: We demonstrate that patients with RF that survived infection had significantly higher IgM, IgG, IgG1 and neutralizing titers compared to patients with RF that succumb to infection, suggesting that using humoral response variables could be used as a prognostic marker for guiding the clinical management of unimmunized patients admitted to the hospital for SARS-CoV-2 infection.
Keywords: COVID disease severity; IgG; IgM 2; SARS-CoV-2; humoral response.
Copyright © 2022 Tajuelo, Carretero, García-Ríos, López-Siles, Cano, Vázquez, Más, Rodríguez-Goncer, Lalueza, López-Medrano, Juan, Fernández-Ruiz, Aguado, McConnell and Pérez-Romero.
Conflict of interest statement
MJM and PPR are founders and stockholders of the biotech-nology spin-off company Vaxdyn, which develops vaccines for infections caused by MDR bacteria. Vaxdyn had no role in the elaboration of this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

