Shh and Olig2 sequentially regulate oligodendrocyte differentiation from hiPSCs for the treatment of ischemic stroke
- PMID: 35547747
- PMCID: PMC9065175
- DOI: 10.7150/thno.69217
Shh and Olig2 sequentially regulate oligodendrocyte differentiation from hiPSCs for the treatment of ischemic stroke
Abstract
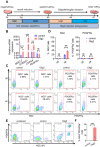
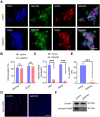
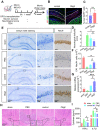
Rationale: Demyelination is a major component of white matter injury, characterized by oligodendrocyte (OL) death and myelin sheath loss, which result in memory loss and cognitive impairment in the context of ischemic stroke. Accumulating evidence has shown that OLs can be generated by the direct activation of defined transcription factors (TFs) in human induced pluripotent stem cells (hiPSCs); however, the rapid acquisition of single TF-induced OL progenitor cells (OPCs) as cell therapy for ischemic stroke remains to be thoroughly explored. Methods: A stable, chemically defined protocol was used to generate a substantial number of transplantable and functional OLs through the partial inhibition of sonic hedgehog (Shh) activity by GANT61 during neural induction from hiPSCs and sequential induction of TF Olig2 overexpression. Transcriptome and metabolome analyses further revealed a novel molecular event in which Olig2 regulates OL differentiation from hiPSC-derived neural progenitor cells (NPCs). Olig2-induced NG2+ OPCs (Olig2-OPCs) were then evaluated for their therapeutic potential in cell-based therapy for ischemic stroke. Results: GANT61 treatment resulted in a motor neuron (MN)-OL fate switch during neural induction, and induced overexpression of Olig2 accelerated oligodendroglial lineage cell specification. Olig2-OPCs expressed typical oligodendroglial lineage marker genes, including NKX2.2, CSPG4, and ST8SIA1, and displayed superior ability to differentiate into mature OLs in vitro. Mechanistically, Olig2-OPCs showed increased gene expression of the peroxisome proliferator-activated receptor γ (PPARγ) signaling pathway, and activated CEPT1-mediated phospholipogenesis. Functionally, inhibiting PPARγ and knocking down CEPT1 further compromised the terminal differentiation of Olig2-OPCs. Most importantly, when transplanted into a rat model of transient middle cerebral artery occlusion (tMCAO), Olig2-OPCs efficiently promoted neurological functional recovery by reducing neuronal death, promoting remyelination, and rescuing spatial memory decline. Conclusions: We developed a stable, chemically defined protocol to generate OPCs/OLs with partial inhibition of Shh activity by GANT61 from hiPSCs and sequentially induced the expression of the single TF Olig2. Olig2-OPC transplantation may be an ideal alternative approach for ischemic stroke rehabilitation therapy.
Keywords: Human induced pluripotent stem cells (hiPSCs); Ischemic stroke.; Olig2; Oligodendrocyte progenitor cells (OPCs); Sonic hedgehog (Shh).
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. - PubMed
-
- Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia. 2010;58:730–40. - PubMed
-
- Nunn J, Hodges H. Cognitive deficits induced by global cerebral ischaemia: relationship to brain damage and reversal by transplants. Behav Brain Res. 1994;65:1–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

