AGK regulates the progression to NASH by affecting mitochondria complex I function
- PMID: 35547757
- PMCID: PMC9065199
- DOI: 10.7150/thno.69826
AGK regulates the progression to NASH by affecting mitochondria complex I function
Abstract
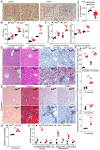
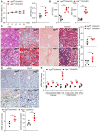
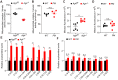
Background: Impaired mitochondrial function contributes to non-alcoholic steatohepatitis (NASH). Acylglycerol kinase (AGK) is a subunit of the translocase of the mitochondrial inner membrane 22 (TIM22) protein import complex. AGK mutation is the leading cause of Sengers syndrome, characterized by congenital cataracts, hypertrophic cardiomyopathy, skeletal myopathy, lactic acidosis, and liver dysfunction. The potential roles and mechanisms of AGK in NASH are not yet elucidated. Methods: Hepatic-specific AGK-deficient mice and AGK G126E mutation (AGK kinase activity arrest) mice were on a choline-deficient and high-fat diet (CDAHFD) and a methionine choline-deficient diet (MCD). The mitochondrial function and the molecular mechanisms underlying AGK were investigated in the pathogenesis of NASH. Results: The levels of AGK were significantly downregulated in human NASH liver samples. AGK deficiency led to severe liver damage and lipid accumulation in mice. Aged mice lacking hepatocyte AGK spontaneously developed NASH. AGK G126E mutation did not affect the structure and function of hepatocytes. AGK deficiency, but not AGK G126E mice, aggravated CDAHFD- and MCD-induced NASH symptoms. AGK deficiency-induced liver damage could be attributed to hepatic mitochondrial dysfunction. The mechanism revealed that AGK interacts with mitochondrial respiratory chain complex I subunits, NDUFS2 and NDUFA10, and regulates mitochondrial fatty acid metabolism. Moreover, the AGK DGK domain might directly interact with NDUFS2 and NDUFA10 to maintain the hepatic mitochondrial respiratory chain complex I function. Conclusions: The current study revealed the critical roles of AGK in NASH. AGK interacts with mitochondrial respiratory chain complex I to maintain mitochondrial integrity via the kinase-independent pathway.
Keywords: NDUFS2; fatty acid metabolism; mitochondrial ROS; mitochondrial respiratory chain.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology. 2016;64:19–22. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G. et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

