Activatable NIR-II organic fluorescent probes for bioimaging
- PMID: 35547762
- PMCID: PMC9065193
- DOI: 10.7150/thno.71359
Activatable NIR-II organic fluorescent probes for bioimaging
Abstract
NIR-II imaging is developed rapidly for noninvasive deep tissue inspection with high spatio-temporal resolution, taking advantage of diminished autofluorescence and light attenuation. Activatable NIR-II fluorescence probes are widely developed to report pathological changes with accurate targeting, among which organic fluorescent probes achieve significant progress. Furthermore, the activatable NIR-II fluorescent probes exhibited appealing characteristics like tunable physicochemical and optical properties, easy processability, and excellent biocompatibility. In the present review, we highlight the advances of activatable NIR-II fluorescence probes in design, synthesis and applications for imaging pathological changes like reactive oxygen species (ROS), reactive nitrogen species (RNS), reactive sulfur species (RSS), pH, hypoxia, viscosity as well as abnormally expressed enzymes. This non-invasive optical imaging modality shows a promising prospect in targeting the pathological site and is envisioned for potential clinical translation.
Keywords: NIR-II fluorescence; bioimaging; organic fluorescent probes; pathological changes; responsive probes.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

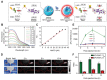


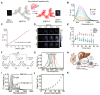


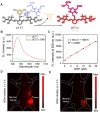

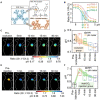




Similar articles
-
Activatable Molecular Probes With Clinical Promise for NIR-II Fluorescent Imaging.Small. 2025 Feb;21(6):e2411787. doi: 10.1002/smll.202411787. Epub 2024 Dec 20. Small. 2025. PMID: 39707663 Review.
-
Activatable Second Near-Infrared Fluorescent Probes: A New Accurate Diagnosis Strategy for Diseases.Biosensors (Basel). 2021 Nov 2;11(11):436. doi: 10.3390/bios11110436. Biosensors (Basel). 2021. PMID: 34821652 Free PMC article. Review.
-
Seeing the unseen: NIR probes for reactive nitrogen species biosensing and bioimaging.Talanta. 2025 Apr 1;285:127334. doi: 10.1016/j.talanta.2024.127334. Epub 2024 Dec 9. Talanta. 2025. PMID: 39673979 Review.
-
Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species.Acc Chem Res. 2019 Sep 17;52(9):2582-2597. doi: 10.1021/acs.accounts.9b00302. Epub 2019 Aug 28. Acc Chem Res. 2019. PMID: 31460742 Free PMC article. Review.
-
Recent progress and outlooks in rhodamine-based fluorescent probes for detection and imaging of reactive oxygen, nitrogen, and sulfur species.Talanta. 2024 Jul 1;274:126004. doi: 10.1016/j.talanta.2024.126004. Epub 2024 Apr 1. Talanta. 2024. PMID: 38564824 Review.
Cited by
-
Recent Advances in the Application of Nitro(het)aromatic Compounds for Treating and/or Fluorescent Imaging of Tumor Hypoxia.Molecules. 2024 Jul 25;29(15):3475. doi: 10.3390/molecules29153475. Molecules. 2024. PMID: 39124883 Free PMC article. Review.
-
Ratiometric fluorescent sensing of pyrophosphate with sp³-functionalized single-walled carbon nanotubes.Nat Commun. 2024 Jan 24;15(1):706. doi: 10.1038/s41467-024-45052-1. Nat Commun. 2024. PMID: 38267487 Free PMC article.
-
Updates on fluorescent probes and open-field imaging methods for fluorescence-guided cytoreductive surgery for epithelial ovarian cancer: A review.BJOG. 2022 Nov;129 Suppl 2(Suppl 2):50-59. doi: 10.1111/1471-0528.17332. BJOG. 2022. PMID: 36485071 Free PMC article. Review.
-
Debut of enzyme-responsive anionic cyanine for overlap-free NIR-II-to-I dual-channel tumour imaging.Chem Sci. 2025 Feb 6;16(10):4490-4500. doi: 10.1039/d4sc06459c. eCollection 2025 Mar 5. Chem Sci. 2025. PMID: 39926710 Free PMC article.
-
(E)-Selective Weinreb Amide-Type Horner-Wadsworth-Emmons Reaction: Effect of Reaction Conditions, Substrate Scope, Isolation of a Reactive Magnesium Phosphonoenolate, and Applications.J Org Chem. 2024 Nov 1;89(21):15414-15435. doi: 10.1021/acs.joc.4c01140. Epub 2024 Oct 11. J Org Chem. 2024. PMID: 39393081 Free PMC article.
References
-
- Liu P, Mu X, Zhang X, Ming D. The near-infrared-II fluorophores and advanced microscopy technologies development and application in bioimaging. Bioconjug Chem. 2020;31:260–275. - PubMed
-
- Yang Q, Ma H, Liang Y, Dai H. Rational design of brightness NIR-II organic dyes with S-D-A-D-S structure. Acc Mater Res. 2021;2:170–83.
-
- Dai H, Ma Z, Wang F, Zhong Y, Salazar F, Li J. et al. Advancing nanomedicine with cross-link functionalized nanoparticles for rapid excretion. Angew Chem Int Ed Engl. 2020;59:1109–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

