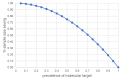
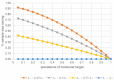
Theranostics approach in drug development: is there study efficiency when the prevalence of the molecular target is very high?
- PMID: 35547778
- PMCID: PMC9065203
- DOI: 10.7150/thno.71788
Theranostics approach in drug development: is there study efficiency when the prevalence of the molecular target is very high?
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- U.S. Food and Drug Administration Guidance for Industry on “Developing Medical Imaging Drug and Biological Products Part 3: Design, Analysis, and interpretation of Clinical Studies”. https://www.fda.gov/regulatory-information/search-fda-guidance-documents....
-
- U.S. FDA Guidance for Industry. Enrichment strategies for clinical trials to support approval of human drugs and biological products. https://www.fda.gov/regulatory-information/search-fda-guidance-documents....
-
- U.S. Food and Drug Administration Companion Diagnostics website. https://www.fda.gov/medical-devices/in-vitro-diagnostics/companion-diagn....
-
- Keytruda (pembrolizumab) injection for intravenous use, highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s120lbl.pdf.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources