Efficacy of ICS versus Non-ICS Combination Therapy in COPD: A Meta-Analysis of Randomised Controlled Trials
- PMID: 35547781
- PMCID: PMC9084385
- DOI: 10.2147/COPD.S347588
Efficacy of ICS versus Non-ICS Combination Therapy in COPD: A Meta-Analysis of Randomised Controlled Trials
Abstract
Background: Several large randomized clinical trials (RCTs) have assessed the efficacy and safety of inhaled corticosteroid (ICS) combination regimens versus non-ICS therapy in patients with chronic obstructive pulmonary disease (COPD) at increased risk of exacerbation risk with mixed results.
Methods: We performed a systematic literature review and meta-analysis of RCTs comparing the effect of ICS-containing combination therapy and non-ICS regimen in patients with COPD.
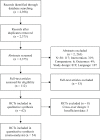
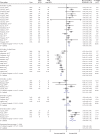
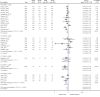
Results: A total of 54 RCTs (N = 57,333) reported treatment effects on various outcomes and were eligible for inclusion. Overall, the number of patients experiencing moderate/severe exacerbations was significantly lower for ICS-containing combination therapy versus non-ICS therapy (RR: 0.86 [95% CI: 0.80-0.93]). The annual rate of exacerbations was also significantly reduced by 22% (0.78 [0.72-0.86]) with ICS-containing versus non-ICS therapy. The annual rate of exacerbations requiring hospitalisation was reduced by 31% versus non-ICS therapy (0.69 [0.54-0.88]); similar reduction was observed for exacerbations requiring oral steroids (0.69 [0.66-0.73]). Overall, the effect on trough FEV1 was comparable between ICS-containing and non-ICS therapies (follow-up: 6-52 weeks); however, a significant improvement in lung function (trough FEV1) was observed for ICS/LABA versus LABA (MD: +0.04 L [0.03-0.05]) and ICS/LABA/LAMA versus LAMA (MD: +0.09 L [0.05-0.13]) regimens. In addition, a significant improvement in QoL was observed with ICS-containing versus non-ICS therapy (MD in SGRQ score: -0.90 [-1.50, -0.31]).
Conclusion: This meta-analysis demonstrated that a wide range of patients with COPD could benefit from dual and triple ICS-containing therapy.
Keywords: chronic obstructive pulmonary disease; dual therapy; exacerbation; inhaled corticosteroid; meta-analysis; triple therapy.
© 2022 Ding et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Comment in
-
The Therapeutic Index as Indicated by Efficacy/Safety Ratio May Be Primarily Assessed by Meta-Analysis of the Efficacy of ICS Combination Therapy for COPD [Letter].Int J Chron Obstruct Pulmon Dis. 2022 Jun 22;17:1453-1454. doi: 10.2147/COPD.S373924. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 35769224 Free PMC article. No abstract available.
References
-
- GOLD-2020-REPORT-ver1.0wms.pdf [Internet]. [cited December 9, 2021]. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0w.... Accessed April 19, 2022.
-
- Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study - PubMed [Internet]. [cited December 9, 2021]. Available from: https://pubmed.ncbi.nlm.nih.gov/24321804/. Accessed April 19, 2022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

