TMPRSS12 Functions in Meiosis and Spermiogenesis and Is Required for Male Fertility in Mice
- PMID: 35547804
- PMCID: PMC9081376
- DOI: 10.3389/fcell.2022.757042
TMPRSS12 Functions in Meiosis and Spermiogenesis and Is Required for Male Fertility in Mice
Abstract
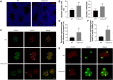
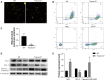
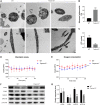
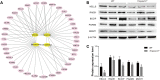
Serine proteases are involved in many physiological activities as initiators of proteolytic cascades, and some members have been reported to play roles in male reproduction. Transmembrane serine protease 12 (TMPRSS12) has been shown to regulate sperm motility and uterotubal junction migration in mice, but its role in the testis remains unknown. In this study, we verified that TMPRSS12 was expressed in the spermatocytes and spermatids of testis and the acrosome of sperm. Mice deficient in Tmprss12 exhibited male sterility. In meiosis, TMPRSS12 was demonstrated to regulate synapsis and double-strand break repair; spermatocytes of Tmprss12 -/- mice underwent impaired meiosis and subsequent apoptosis, resulting in reduced sperm counts. During spermiogenesis, TMPRSS12 was found to function in the development of mitochondria; abnormal mitochondrial structure in Tmprss12 -/- sperm led to reduced availability of ATP, impacting sperm motility. The differential protein expression profiles of testes in Tmprss12 -/- and wild-type mice and further molecule identification revealed potential targets of TMPRSS12 related to meiosis and mitochondrial function. Besides, TMPRSS12 was also found to be involved in a series of sperm functions, including capacitation, acrosome reaction and sperm-egg interaction. These data imply that TMPRSS12 plays a role in multiple aspects of male reproduction.
Keywords: TMPRSS12; male infertility; meiosis; serine protease; spermiogenesis.
Copyright © 2022 Zhang, Zhou, Wan, Yu, Chen, Yan, Wu, Zheng, Zhu and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Akhmanova A., Mausset-Bonnefont A.-L., van Cappellen W., Keijzer N., Hoogenraad C. C., Stepanova T. (2005). The Microtubule Plus-End-Tracking Protein CLIP-170 Associates with the Spermatid Manchette and Is Essential for Spermatogenesis. Genes Dev. 19, 2501–2515. 10.1101/gad.344505 - DOI - PMC - PubMed
-
- Barrett A. J. (2004). Bioinformatics of Proteases in the MEROPS Database. Curr. Opin. Drug Discov. Devel 7, 334–341. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

