Homozygous Loss of Septin12, but not its Haploinsufficiency, Leads to Male Infertility and Fertilization Failure
- PMID: 35547809
- PMCID: PMC9082362
- DOI: 10.3389/fcell.2022.850052
Homozygous Loss of Septin12, but not its Haploinsufficiency, Leads to Male Infertility and Fertilization Failure
Abstract
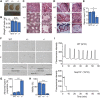
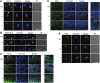
The SEPTIN12 gene has been associated with male infertility. Male Septin12 +/- chimera mice were infertile, supporting the prevailing view that SEPTIN12 haploinsufficiency causes male infertility. In this study, we identified a heterozygous mutation on SEPTIN12, c.72C>A (p.Cys24Ter) in the male partner of a patient couple, who had a previous fertilization failure (FF) after intracytoplasmic sperm injection (ICSI) and became pregnant after ICSI together with artificial oocyte activation (AOA). To investigate the role of SEPTIN12 in FF and oocyte activation, we constructed Septin12 knockout mice. Surprisingly, Septin12 -/- male mice, but not Septin12 +/- male mice, are infertile, and have reduced sperm counts and abnormal sperm morphology. Importantly, AOA treatment enhances the 2-cell embryo rate of ICSI embryos injected with Septin12 -/- sperm, indicating that FF caused by male Septin12 deficiency is overcome by AOA. Mechanistically, loss of PLCζ around the acrosome might be the reason for FF of Septin12 -/- sperm. Taken together, our data indicated that homozygous knockout of Septin12, but not Septin12 haploinsufficiency, leads to male infertility and FF.
Keywords: SEPTIN12; calcium oscillation; fertilization failure; male infertility; oocyte activation.
Copyright © 2022 Chen, Li, Du, Zhao, Wang, Tian, Song, Shuai, Bai and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Escoffier J., Lee H. C., Yassine S., Zouari R., Martinez G., Karaouzène T., et al. (2016). Homozygous Mutation of PLCZ1 Leads to Defective Human Oocyte Activation and Infertility that Is Not Rescued by the WW-Binding Protein PAWP. Hum. Mol. Genet. 25 (5), 878–891. 10.1093/hmg/ddv617 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

