Historical Perspective of Pore-Forming Activity Studies of Voltage-Dependent Anion Channel (Eukaryotic or Mitochondrial Porin) Since Its Discovery in the 70th of the Last Century
- PMID: 35547863
- PMCID: PMC9083909
- DOI: 10.3389/fphys.2021.734226
Historical Perspective of Pore-Forming Activity Studies of Voltage-Dependent Anion Channel (Eukaryotic or Mitochondrial Porin) Since Its Discovery in the 70th of the Last Century
Abstract
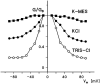
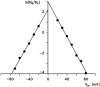
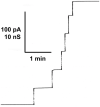
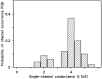
Eukaryotic porin, also known as Voltage-Dependent Anion Channel (VDAC), is the most frequent protein in the outer membrane of mitochondria that are responsible for cellular respiration. Mitochondria are most likely descendants of strictly aerobic Gram-negative bacteria from the α-proteobacterial lineage. In accordance with the presumed ancestor, mitochondria are surrounded by two membranes. The mitochondrial outer membrane contains besides the eukaryotic porins responsible for its major permeability properties a variety of other not fully identified channels. It encloses also the TOM apparatus together with the sorting mechanism SAM, responsible for the uptake and assembly of many mitochondrial proteins that are encoded in the nucleus and synthesized in the cytoplasm at free ribosomes. The recognition and the study of electrophysiological properties of eukaryotic porin or VDAC started in the late seventies of the last century by a study of Schein et al., who reconstituted the pore from crude extracts of Paramecium mitochondria into planar lipid bilayer membranes. Whereas the literature about structure and function of eukaryotic porins was comparatively rare during the first 10years after the first study, the number of publications started to explode with the first sequencing of human Porin 31HL and the recognition of the important function of eukaryotic porins in mitochondrial metabolism. Many genomes contain more than one gene coding for homologs of eukaryotic porins. More than 100 sequences of eukaryotic porins are known to date. Although the sequence identity between them is relatively low, the polypeptide length and in particular, the electrophysiological characteristics are highly preserved. This means that all eukaryotic porins studied to date are anion selective in the open state. They are voltage-dependent and switch into cation-selective substates at voltages in the physiological relevant range. A major breakthrough was also the elucidation of the 3D structure of the eukaryotic pore, which is formed by 19 β-strands similar to those of bacterial porin channels. The function of the presumed gate an α-helical stretch of 20 amino acids allowed further studies with respect to voltage dependence and function, but its exact role in channel gating is still not fully understood.
Keywords: VDAC; electrophysiology; eukaryotic pore; evolution; mitochondria; porin; single channel.
Copyright © 2021 Benz.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Solute Transport through Mitochondrial Porins In Vitro and In Vivo.Biomolecules. 2024 Mar 4;14(3):303. doi: 10.3390/biom14030303. Biomolecules. 2024. PMID: 38540723 Free PMC article. Review.
-
Studies on human porin. VII. The channel properties of the human B-lymphocyte membrane-derived "Porin 31HL" are similar to those of mitochondrial porins.Biol Chem Hoppe Seyler. 1992 Jun;373(6):295-303. doi: 10.1515/bchm3.1992.373.1.295. Biol Chem Hoppe Seyler. 1992. PMID: 1381184
-
Biophysical properties of porin pores from mitochondrial outer membrane of eukaryotic cells.Experientia. 1990 Feb 15;46(2):131-7. doi: 10.1007/BF02027308. Experientia. 1990. PMID: 1689250 Review.
-
A 3D model of the voltage-dependent anion channel (VDAC).FEBS Lett. 2002 Jun 5;520(1-3):1-7. doi: 10.1016/s0014-5793(02)02758-8. FEBS Lett. 2002. PMID: 12044860
-
Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane.Ann N Y Acad Sci. 1980;341:552-63. doi: 10.1111/j.1749-6632.1980.tb47198.x. Ann N Y Acad Sci. 1980. PMID: 6249159
Cited by
-
Developing a Novel and Optimized Yeast Model for Human VDAC Research.Int J Mol Sci. 2024 Dec 3;25(23):13010. doi: 10.3390/ijms252313010. Int J Mol Sci. 2024. PMID: 39684721 Free PMC article.
-
Solute Transport through Mitochondrial Porins In Vitro and In Vivo.Biomolecules. 2024 Mar 4;14(3):303. doi: 10.3390/biom14030303. Biomolecules. 2024. PMID: 38540723 Free PMC article. Review.
-
Beta-Barrel Channel Response to High Electric Fields: Functional Gating or Reversible Denaturation?Int J Mol Sci. 2023 Nov 23;24(23):16655. doi: 10.3390/ijms242316655. Int J Mol Sci. 2023. PMID: 38068977 Free PMC article.
-
How to isolate channel-forming membrane proteins using the E. coli expression system.Nat Protoc. 2025 Feb;20(2):462-479. doi: 10.1038/s41596-024-01055-2. Epub 2024 Oct 4. Nat Protoc. 2025. PMID: 39367089 Review.
-
Tracking the Activity and Position of Mitochondrial β-Barrel Proteins.Methods Mol Biol. 2024;2778:221-236. doi: 10.1007/978-1-0716-3734-0_14. Methods Mol Biol. 2024. PMID: 38478281
References
-
- Aiello R., Messina A., Schiffler B., Benz R., Tasco G., Casadio R., et al. . (2004). Functional characterization of a second porin isoform in Drosophila melanogaster. DmPorin2 forms voltage-independent cation-selective pores. J. Biol. Chem. 279, 25364–25373. doi: 10.1074/jbc.M310572200, PMID: - DOI - PubMed
-
- Anflous K., Craigen W. J. (2004). “Mitochondrial porins in mammals: insight into functional roles from mutant mice and cells,” in Structure and Function of Prokaryotic and Eukaryotic Porins. ed. Benz R. (Wiley-VCh: Weinheim; ), 285–307.
Publication types
LinkOut - more resources
Full Text Sources

