Intra-Tumoral CD8+ T-Cell Infiltration and PD-L1 Positivity in Homologous Recombination Deficient Pancreatic Ductal Adenocarcinoma
- PMID: 35547873
- PMCID: PMC9082359
- DOI: 10.3389/fonc.2022.860767
Intra-Tumoral CD8+ T-Cell Infiltration and PD-L1 Positivity in Homologous Recombination Deficient Pancreatic Ductal Adenocarcinoma
Abstract
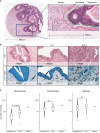
The immune contexture of pancreatic ductal adenocarcinoma (PDAC) is generally immunosuppressive. A role for immune checkpoint inhibitors (ICIs) in PDAC has only been demonstrated for the rare and hypermutated mismatch repair (MMR) deficient (MMR-d) subtype. Homologous recombination repair (HR) deficient (HR-d) PDAC is more prevalent and may encompass up to 20% of PDAC. Its genomic instability may promote a T-cell mediated anti-tumor response with therapeutic sensitivity to ICIs. To investigate the immunogenicity of HR-d PDAC, we used multiplex immunohistochemistry (IHC) to compare the density and spatial distribution of CD8+ cytotoxic T-cells, FOXP3+ regulatory T-cells (Tregs), and CD68+ tumor-associated macrophages (TAMs) in HR-d versus HR/MMR-intact PDAC. We also evaluated the IHC positivity of programmed death-ligand 1 (PD-L1) across the subgroups. 192 tumors were evaluated and classified as HR/MMR-intact (n=166), HR-d (n=25) or MMR-d (n=1) based on germline testing and tumor molecular hallmarks. Intra-tumoral CD8+ T-cell infiltration was higher in HR-d versus HR/MMR-intact PDAC (p<0.0001), while CD8+ T-cell densities in the peri-tumoral and stromal regions were similar in both groups. HR-d PDAC also displayed increased intra-tumoral FOXP3+ Tregs (p=0.049) and had a higher CD8+:FOXP3+ ratio (p=0.023). CD68+ TAM expression was similar in HR-d and HR/MMR-intact PDAC. Finally, 6 of the 25 HR-d cases showed a PD-L1 Combined Positive Score of >=1, whereas none of the HR/MMR-intact cases met this threshold (p<0.00001). These results provide immunohistochemical evidence for intra-tumoral CD8+ T-cell enrichment and PD-L1 positivity in HR-d PDAC, suggesting that HR-d PDAC may be amenable to ICI treatment strategies.
Keywords: PD-L1; T-cell inflammation; immunotherapy; pancreatic cancer; tumor microenvironment.
Copyright © 2022 Golesworthy, Wang, Tanti, Pacis, Romero, Cuggia, Domecq, Bourdel, Denroche, Jang, Grant, Borgida, Grünwald, Dodd, Wilson, Bourque, O’Kane, Fischer, Kron, Fiset, Omeroglu, Foulkes, Gallinger, Guiot, Gao and Zogopoulos.
Conflict of interest statement
P-OF has received honoraria from EMD Serono and consultation fees from Amgen, Bristol Myers Squibb, AstraZeneca Canada, Hoffmann La Roche, Merck Canada, Pfizer Canada and Roche Canada. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ferlay J EM, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, et al. . Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. (2020). Available at: https://gco.iarc.fr/today.
-
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. . Comparison of Adjuvant Gemcitabine and Capecitabine With Gemcitabine Monotherapy in Patients With Resected Pancreatic Cancer (ESPAC-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet (2017) 389(10073):1011–24. doi: 10.1016/S0140-6736(16)32409-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

