Mechanistic study of the ligand controlled regioselectivity in iridium catalyzed C-H borylation of aromatic imines
- PMID: 35547887
- PMCID: PMC9088018
- DOI: 10.1039/c8ra07886f
Mechanistic study of the ligand controlled regioselectivity in iridium catalyzed C-H borylation of aromatic imines
Abstract
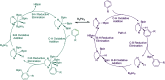
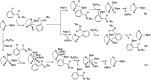
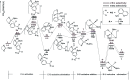
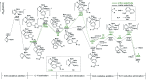
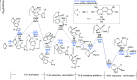
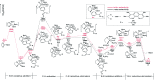
As a major challenge in C-H borylation, how to control the selectivity has attracted lots of attention, however, the related mechanistic information still needs to be uncovered. Herein, density functional theory (DFT) has been used to study the mechanism for the ligand controlled regioselectivity in the iridium-catalyzed C-H borylation of aromatic imines, which is inspired by experimental observations (R. Bisht, B. Chattopadhyay, J. Am. Chem. Soc., 2016, 138, 84-87). The proposed Ir(i)-Ir(iii) catalytic cycle includes (i) the oxidative addition of the C-H bond to iridium(i); (ii) the reductive elimination of a C-B bond; (iii) the oxidative addition of B2pin2 to an iridium(i) hydride complex; and (iv) the reductive elimination of a B-H bond. The oxidative addition of a C-H bond to the iridium center is the determining step. For the ligand AQ, ortho-selectivity is proposed to be attributed to the decreased steric hindrance and increased electron donating effect of AQ (8-aminoquinoline) which promotes proton-transfer in the ortho-transition state of C-H activation. While, for the TMP ligand, the steric repulsion between the TMP (4,5,7,8-tetramethyl-1, 10-phenanthroline) ligand and the ortho-substituted imine hinders the ortho C-H activation and favors meta borylation. Our calculations provide insights into further ligand design to achieve different regioselective borylation of aromatics. Guided by the results, the regioselectivity in the borylation of aromatics may be achieved by accordingly modifying the electronic and steric substituents of the ligand.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Computational understanding of catalyst-controlled borylation of fluoroarenes: directed vs. undirected pathway.RSC Adv. 2020 May 21;10(33):19562-19569. doi: 10.1039/d0ra03428b. eCollection 2020 May 20. RSC Adv. 2020. PMID: 35515481 Free PMC article.
-
Iridium-Catalyzed Regioselective Borylation through C-H Activation and the Origin of Ligand-Dependent Regioselectivity Switching.J Org Chem. 2021 Nov 5;86(21):15618-15630. doi: 10.1021/acs.joc.1c02126. Epub 2021 Oct 1. J Org Chem. 2021. PMID: 34598435
-
sp3 C-H Borylation Catalyzed by Iridium(III) Triboryl Complex: Comprehensive Theoretical Study of Reactivity, Regioselectivity, and Prediction of Excellent Ligand.J Am Chem Soc. 2019 Jun 26;141(25):9854-9866. doi: 10.1021/jacs.9b01767. Epub 2019 Jun 17. J Am Chem Soc. 2019. PMID: 31124356
-
Iridium-Catalysed C-H Borylation of Heteroarenes: Balancing Steric and Electronic Regiocontrol.Angew Chem Int Ed Engl. 2021 Feb 8;60(6):2796-2821. doi: 10.1002/anie.202001520. Epub 2020 Nov 3. Angew Chem Int Ed Engl. 2021. PMID: 32202024 Free PMC article. Review.
-
Ir-catalyzed proximal and distal C-H borylation of arenes.Chem Commun (Camb). 2021 Dec 7;57(97):13059-13074. doi: 10.1039/d1cc05104k. Chem Commun (Camb). 2021. PMID: 34782892 Review.
Cited by
-
Interrogating the Crucial Interactions at Play in the Chiral Cation-Directed Enantioselective Borylation of Arenes.ACS Catal. 2023 Sep 22;13(19):13043-13055. doi: 10.1021/acscatal.3c03384. eCollection 2023 Oct 6. ACS Catal. 2023. PMID: 37822864 Free PMC article.
-
Computational understanding of catalyst-controlled borylation of fluoroarenes: directed vs. undirected pathway.RSC Adv. 2020 May 21;10(33):19562-19569. doi: 10.1039/d0ra03428b. eCollection 2020 May 20. RSC Adv. 2020. PMID: 35515481 Free PMC article.
References
-
- Ros A. Fernandez R. Lassaletta J. M. Chem. Soc. Rev. 2014;43:3229. doi: 10.1039/C3CS60418G. - DOI - PubMed
- Hartwig J. F. J. Am. Chem. Soc. 2016;138:2. doi: 10.1021/jacs.5b08707. - DOI - PMC - PubMed
- Xu L. Wang G. Zhang S. Wang H. Wang L. Liu L. Jiao J. Li P. Tetrahedron. 2017;73:7123. doi: 10.1016/j.tet.2017.11.005. - DOI
- Mkhalid I. Barnard J. H. Marder T. B. Murphy J. M. Hartwig J. F. Chem. Rev. 2010;110:890. doi: 10.1021/cr900206p. - DOI - PubMed
- Hall D. G., Wiley-VCH: Weinheim, Germany, 2005
- Dick G. R. Woerly E. M. Burke M. D. Angew. Chem., Int. Ed. 2012;51:2667. doi: 10.1002/anie.201108608. - DOI - PMC - PubMed
-
- Xue C. Luo Y. Teng H. L. Ma Y. H. Nishiura M. Hou Z. M. ACS Catal. 2018;8:5017. doi: 10.1021/acscatal.8b01364. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

