Theoretical and experimental investigation of anticancer activities of an acyclic and symmetrical compartmental Schiff base ligand and its Co(ii), Cu(ii) and Zn(ii) complexes
- PMID: 35547928
- PMCID: PMC9088086
- DOI: 10.1039/c8ra07463a
Theoretical and experimental investigation of anticancer activities of an acyclic and symmetrical compartmental Schiff base ligand and its Co(ii), Cu(ii) and Zn(ii) complexes
Abstract
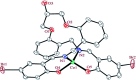
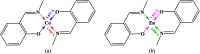
A compartmental Schiff base ligand, 2,2'-((((((2-hydroxypropane-1,3-diyl)bis(oxy))bis(2,1-phenylene))bis(methylene))bis(azanylylidene))bis(methanylylidene))bis(4-bromophenol) (H3LBr) and its complexes with cobalt(ii), copper(ii) and zinc(ii) including, [Co(HLBr)] (1), [Cu2(LBr)(μ-1,3-OAc)]·MeOH (2) and [Zn(HLBr)] (3) were prepared using template synthesis and characterised by elemental analysis, FT-IR and 1H NMR spectroscopies and single-crystal X-ray diffraction. In the structure of complexes 1 and 3 the metal atom has a MN2O2 environment with tetrahedral geometry while complex 2 has a binuclear structure with a MNO4 environment and square planar geometry around the copper atom. The ability of all compounds to interact with the nine biomacromolecules (BRAF kinase, CatB, DNA gyrase, HDAC7, rHA, RNR, TrxR, TS and Top II) are investigated by docking calculations. For examination of the docking results, the in vitro activities of eight compounds against the human leukemia cell line K562 was investigated by evaluation of IC50 values and mode of cell death (apoptosis).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









References
-
- Gupta K. C. Sutar A. K. Coord. Chem. Rev. 2008;252:1420–1450. doi: 10.1016/j.ccr.2007.09.005. - DOI
-
- Kannappan R. Matsumoto M. Hallren J. Nicholas K. M. J. Mol. Catal. A: Chem. 2011;339:72–78. doi: 10.1016/j.molcata.2011.02.014. - DOI
-
- Li L.-H. Dong W.-K. Zhang Y. Akogun S. F. Xu L. Appl. Organomet. Chem. 2017;31:e3818. doi: 10.1002/aoc.3818. - DOI
-
- Wesley Jeevadason A. Kalidasa Murugavel K. Neelakantan M. A. Renewable Sustainable Energy Rev. 2014;36:220–227. doi: 10.1016/j.rser.2014.04.060. - DOI
-
- Dong W.-K. Li X.-L. Wang L. Zhang Y. Ding Y.-J. Sens. Actuators. 2016;B229:370–378. doi: 10.1016/j.snb.2016.01.139. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

