High electrochemical performance of nanocrystallized carbon-coated LiFePO4 modified by tris(pentafluorophenyl) borane as a cathode material for lithium-ion batteries
- PMID: 35547964
- PMCID: PMC9084481
- DOI: 10.1039/c8ra04119a
High electrochemical performance of nanocrystallized carbon-coated LiFePO4 modified by tris(pentafluorophenyl) borane as a cathode material for lithium-ion batteries
Abstract
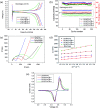
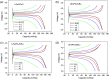
Tris(pentafluorophenyl) borane (C18BF15) was first adopted as a boron source, which clearly demonstrated its modification effects. XPS and EDX mapping proved that boron can be successfully doped into a carbon layer. The high number of defects in the carbon induced by boron was demonstrated via Raman spectroscopy and thus, the electric conductivity of LiFePO4 was greatly enhanced. The boron-doped composite possessed a higher specific discharge capacity and rate capability than the undoped sample. For instance, the reversible specific capacity for the boron-doped cathode reached 165.8 mA h g-1 at 0.5C, which was almost close to its theoretical capacity (166 mA h g-1). Even at a high rate of 5C, it still possessed a high specific capacity of 124.8 mA h g-1. This provides for the possibility that boron-doped carbon-coated LiFePO4 cathodes may deliver high energy and power density for rechargeable lithium-ion batteries.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




Similar articles
-
Boron and Nitrogen Codoped Carbon Layers of LiFePO4 Improve the High-Rate Electrochemical Performance for Lithium Ion Batteries.ACS Appl Mater Interfaces. 2015 Sep 16;7(36):20134-43. doi: 10.1021/acsami.5b05398. Epub 2015 Sep 2. ACS Appl Mater Interfaces. 2015. PMID: 26305802
-
Insight into the effect of boron doping on sulfur/carbon cathode in lithium-sulfur batteries.ACS Appl Mater Interfaces. 2014 Jun 11;6(11):8789-95. doi: 10.1021/am501627f. Epub 2014 Apr 24. ACS Appl Mater Interfaces. 2014. PMID: 24764111
-
Preparation of Nb5+ Doped Na3V2(PO4)3 Cathode Material for Sodium Ion Batteries.Materials (Basel). 2024 Jun 3;17(11):2697. doi: 10.3390/ma17112697. Materials (Basel). 2024. PMID: 38893960 Free PMC article.
-
Superior electrochemical performance of a novel LiFePO4/C/CNTs composite for aqueous rechargeable lithium-ion batteries.Phys Chem Chem Phys. 2020 Jan 28;22(4):1953-1962. doi: 10.1039/c9cp06042a. Epub 2020 Jan 15. Phys Chem Chem Phys. 2020. PMID: 31939949
-
Preparation of V-Doped LiFePO4/C as the Optimized Cathode Material for Lithium Ion Batteries.J Nanosci Nanotechnol. 2015 Apr;15(4):2667-72. doi: 10.1166/jnn.2015.9149. J Nanosci Nanotechnol. 2015. PMID: 26353479
References
-
- Meng Y. Xia J. Wang L. Wang G. Zhu F. Zhang Y. A comparative study on LiFePO4/C by in situ coating with different carbon sources for high-performance lithium batteries. Electrochim. Acta. 2018;261:96–103. doi: 10.1016/j.electacta.2017.12.127. - DOI
-
- Wang X. Feng Z. Huang J. Deng W. Li X. Zhang H. Wen Z. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries. Carbon. 2018;127:149–157. doi: 10.1016/j.carbon.2017.10.101. - DOI
-
- Raj H. Sil A. Effect of carbon coating on electrochemical performance of LiFePO4 cathode material for Li-ion battery. Ionics. 2018 doi: 10.1007/s11581-017-2423-0. - DOI
-
- Wang J. J. Sun X. L. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012;5:5163–5185. doi: 10.1039/C1EE01263K. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

