Photo-responsive polymeric micelles and prodrugs: synthesis and characterization
- PMID: 35547974
- PMCID: PMC9084478
- DOI: 10.1039/c8ra04580a
Photo-responsive polymeric micelles and prodrugs: synthesis and characterization
Abstract
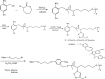
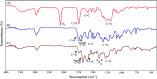
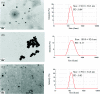
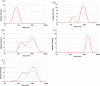
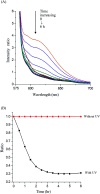
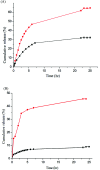
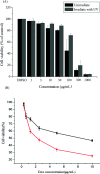
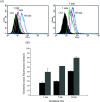
Bio-recognizable and photocleavable amphiphilic glycopolymers and prodrugs containing photodegradable linkers (i.e. 5-hydroxy-2-nitrobenzyl alcohol) as junction points between bio-recognizable hydrophilic glucose (or maltose) and hydrophobic poly(α-azo-ε-caprolactone)-grafted alkyne or drug chains were synthesized by combining ring-opening polymerization, nucleophilic substitution, and "click" post-functionalization with alkynyl-pyrene and 2-nitrobenzyl-functionalized indomethacin (IMC). The block-grafted glycocopolymers could self-assemble into spherical photoresponsive micelles with hydrodynamic sizes of <200 nm. Fluorescence emission measurements indicated the release of Nile red, a hydrophobic dye, encapsulated by the Glyco-ONB-P(αN3CL-g-alkyne) n micelles, in response to irradiation caused by micelle disruption. Light-triggered bursts were observed for IMC-loaded or -conjugated micelles during the first 5 h. Following light irradiation, the drug release rate of IMC-conjugated micelles was faster than that of IMC-loaded micelles. Selective lectin binding experiments confirmed that glycosylated Glyco-ONB-P(αN3CL-g-alkyne) n could be used in bio-recognition applications. The nano-prodrug with and without UV irradiation was associated with negligible levels of toxicity at concentrations of less than 30 μg mL-1. The confocal microscopy and flow cytometry results indicated that the uptake of doxorubicin (DOX)-loaded micelles with UV irradiation by HeLa cells was faster than without UV irradiation. The DOX-loaded Gluco-ONB-P(αN3CL-g-PONBIMC)10 micelles effectively inhibited HeLa cells' proliferation with a half-maximal inhibitory concentration of 8.8 μg mL-1.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








Similar articles
-
Synthesis and characterization of amphiphilic photocleavable polymers based on dextran and substituted-ɛ-caprolactone.Carbohydr Polym. 2015 Mar 6;117:201-210. doi: 10.1016/j.carbpol.2014.09.062. Epub 2014 Oct 2. Carbohydr Polym. 2015. PMID: 25498626
-
Photo- and pH-Responsive Polycarbonate Block Copolymer Prodrug Nanomicelles for Controlled Release of Doxorubicin.Macromol Biosci. 2020 Aug;20(8):e2000118. doi: 10.1002/mabi.202000118. Epub 2020 Jun 22. Macromol Biosci. 2020. PMID: 32567108
-
One-Pot Synthesis of pH/Redox Responsive Polymeric Prodrug and Fabrication of Shell Cross-Linked Prodrug Micelles for Antitumor Drug Transportation.Bioconjug Chem. 2018 Aug 15;29(8):2806-2817. doi: 10.1021/acs.bioconjchem.8b00421. Epub 2018 Jul 30. Bioconjug Chem. 2018. PMID: 30005157
-
Doxorubicin-loaded nanosized micelles of a star-shaped poly(ε-caprolactone)-polyphosphoester block co-polymer for treatment of human breast cancer.J Biomater Sci Polym Ed. 2011;22(11):1409-26. doi: 10.1163/092050610X510533. Epub 2010 Jun 30. J Biomater Sci Polym Ed. 2011. PMID: 20594418
-
Fine tuning micellar core-forming block of poly(ethylene glycol)-block-poly(ε-caprolactone) amphiphilic copolymers based on chemical modification for the solubilization and delivery of doxorubicin.Biomacromolecules. 2011 Jul 11;12(7):2562-72. doi: 10.1021/bm200375x. Epub 2011 Jun 6. Biomacromolecules. 2011. PMID: 21598958
Cited by
-
Dual Thermo- and Photo-Responsive Micelles Based on Azobenzene-Containing Random Copolymer.Materials (Basel). 2021 Dec 21;15(1):2. doi: 10.3390/ma15010002. Materials (Basel). 2021. PMID: 35009149 Free PMC article.
References
-
- Aoleimani A. Borecki A. Gillies E. R. Polym. Chem. 2014;5:7062–7071. doi: 10.1039/C4PY00996G. - DOI
LinkOut - more resources
Full Text Sources

