Preparation of triangular silver nanoplates by silver seeds capped with citrate-CTA
- PMID: 35547977
- PMCID: PMC9084414
- DOI: 10.1039/c8ra04554b
Preparation of triangular silver nanoplates by silver seeds capped with citrate-CTA
Abstract
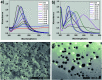
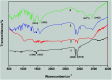
Due to the competitive growth on the crystal face of seed, it is always difficult to control the morphology of the formation of nanoparticles precisely by a seed-mediated growth method. Herein, we provided a simple but effective technique to synthesize silver nanotriangles using a new silver seed that is capped with citrate-CTA+ (CTA+ is cetyltrimethyl ammonium cation). Compared to the preparation of silver nanoparticles (AgNPs) by a conventional seed-mediated method, in this paper, we presented a growth technique with two distinct innovative changes. First, the concentrations of CTAB that we added in silver seed collosol have a significant impact on the size distribution, and silver nanotriangles, nanorods, and nanospheres could be obtained by adjusting the CTAB concentration. Second, the seed prepared by our method has a longer use time, and silver nanotriangles, nanospheres, and nanorods could be prepared by adjusting the aged time of the seed colloid. We have also shown a simple way to control the morphology of silver nanoparticles in almost the same reactive medium by varying the NaOH concentration. Using the new silver seed capped with citrate-CTA+, we obtained triangular silver nanoparticles with relatively high regularity. Based on the limited experimental results and IR analysis, a possible mechanism was preliminarily proposed to explain the formation of the seed and the truncated triangular AgNPs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
The Influence of CTAB-Capped Seeds and Their Aging Time on the Morphologies of Silver Nanoparticles.Nanoscale Res Lett. 2019 Mar 5;14(1):81. doi: 10.1186/s11671-019-2898-x. Nanoscale Res Lett. 2019. PMID: 30838472 Free PMC article.
-
Quasi-spherical silver nanoparticles: aqueous synthesis and size control by the seed-mediated Lee-Meisel method.J Colloid Interface Sci. 2013 Mar 15;394:263-8. doi: 10.1016/j.jcis.2012.12.037. Epub 2012 Dec 28. J Colloid Interface Sci. 2013. PMID: 23332939
-
Light emitting diode irradiation can control the morphology and optical properties of silver nanoparticles.J Am Chem Soc. 2010 Feb 17;132(6):1825-7. doi: 10.1021/ja910010b. J Am Chem Soc. 2010. PMID: 20102152
-
Influence of Temperature on the Formation of Silver Nanoparticles by using a Seed-Free Photochemical Method under Sodium-Lamp Irradiation.Chemphyschem. 2015 Oct 26;16(15):3254-63. doi: 10.1002/cphc.201500485. Epub 2015 Sep 11. Chemphyschem. 2015. PMID: 26269109
-
Rapid, reversible preparation of size-controllable silver nanoplates by chemical redox.Langmuir. 2010 Jul 20;26(14):11621-3. doi: 10.1021/la1016627. Langmuir. 2010. PMID: 20550181
Cited by
-
Tailoring innovative silver nanoparticles for modern medicine: The importance of size and shape control and functional modifications.Mater Today Bio. 2025 Jul 9;33:102071. doi: 10.1016/j.mtbio.2025.102071. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40727080 Free PMC article. Review.
-
Ag nanorod@PEI-Ag nanohybrid as an excellent signal label for sensitive and rapid detection of serum HER2.Sci Rep. 2023 Dec 8;13(1):21792. doi: 10.1038/s41598-023-48838-3. Sci Rep. 2023. PMID: 38066021 Free PMC article.
-
Preparation of Fe3O4-Ag Nanocomposites with Silver Petals for SERS Application.Nanomaterials (Basel). 2021 May 13;11(5):1288. doi: 10.3390/nano11051288. Nanomaterials (Basel). 2021. PMID: 34068287 Free PMC article.
-
The Influence of CTAB-Capped Seeds and Their Aging Time on the Morphologies of Silver Nanoparticles.Nanoscale Res Lett. 2019 Mar 5;14(1):81. doi: 10.1186/s11671-019-2898-x. Nanoscale Res Lett. 2019. PMID: 30838472 Free PMC article.
References
LinkOut - more resources
Full Text Sources

