Dietary Supplementation of Ferrous Glycine Chelate Improves Growth Performance of Piglets by Enhancing Serum Immune Antioxidant Properties, Modulating Microbial Structure and Its Metabolic Function in the Early Stage
- PMID: 35548055
- PMCID: PMC9083199
- DOI: 10.3389/fvets.2022.876965
Dietary Supplementation of Ferrous Glycine Chelate Improves Growth Performance of Piglets by Enhancing Serum Immune Antioxidant Properties, Modulating Microbial Structure and Its Metabolic Function in the Early Stage
Abstract
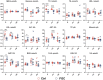
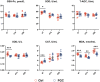
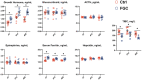
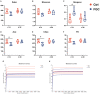
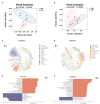
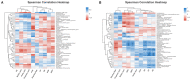
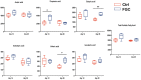
The present research aimed to explore the effect of dietary ferrous glycine chelate supplementation on performance, serum immune-antioxidant parameters, fecal volatile fatty acids, and microbiota in weaned piglets. A total of 80 healthy piglets (weaned at 28 day with an initial weight of 7.43 ± 1.51 kg) were separated into two treatments with five replicates of eight pigs each following a completely randomized block design. The diet was a corn-soybean basal diet with 2,000 mg/kg ferrous glycine chelates (FGC) or not (Ctrl). The serum and fecal samples were collected on days 14 and 28 of the experiment. The results indicated that dietary FGC supplementation improved (p < 0.05) the average daily gain and average daily feed intake overall, alleviated (p < 0.05) the diarrhea rate of piglets at the early stage, enhanced (p < 0.05) the levels of superoxide dismutase and catalase on day 14 and lowered (p < 0.05) the MDA level overall. Similarly, the levels of growth hormone and serum iron were increased (p < 0.05) in the FGC group. Moreover, dietary FGC supplementation was capable of modulating the microbial community structure of piglets in the early period, increasing (p < 0.05) the abundance of short-chain fatty acid-producing bacteria Tezzerella, decreasing (p < 0.05) the abundance of potentially pathogenic bacteria Slackia, Olsenella, and Prevotella as well as stimulating (p < 0.05) the propanoate and butanoate metabolisms. Briefly, dietary supplemented FGC ameliorates the performance and alleviated the diarrhea of piglets by enhancing antioxidant properties, improving iron transport, up-regulating the growth hormone, modulating the fecal microbiota, and increasing the metabolism function. Therefore, FGC is effective for early iron supplementation and growth of piglets and may be more effective in neonatal piglets.
Keywords: antioxidative property; fecal microbiota; ferrous glycine chelate; growth performance; piglets.
Copyright © 2022 Ma, Liu, Piao, Wang, Wang, Lin, Hsu and Liu.
Conflict of interest statement
Y-SL and T-PH were employed by Shanghai Bestar biochemical Co. Ltd. LL were employed by Tianjin Zhongsheng Feed Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

