Asymmetric vinylogous aldol addition of alkylidene oxindoles on trifluoromethyl-α,β-unsaturated ketones
- PMID: 35548158
- PMCID: PMC9086480
- DOI: 10.1039/c8ra06615a
Asymmetric vinylogous aldol addition of alkylidene oxindoles on trifluoromethyl-α,β-unsaturated ketones
Abstract
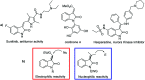
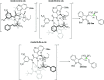
A novel vinylogous aldol addition of alkylidene oxindole with 1-trifluoromethyl-3-alkylidene-propan-2-ones is presented. The reaction, catalyzed by a bifunctional tertiary amine, provides an efficient application of the vinylogous reactivity of oxindoles for the preparation of enantioenriched trifluoromethylated allylic alcohols.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
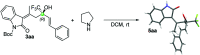
References
-
- Fuson R. C. Chem. Rev. 1935;16:1. doi: 10.1021/cr60053a001. - DOI
- Battisitini L. Synthesis. 2017;49:2297. doi: 10.1055/s-0036-1589487. - DOI
- Jurberg I. D. Chatterjee I. Tannert R. Melchiorre P. Chem. Commun. 2013;49:4869. doi: 10.1039/C3CC41270A. - DOI - PubMed
- Casiraghi G. Battistini L. Curti C. Rassu G. Zanardi F. Chem. Rev. 2011;111:3076. doi: 10.1021/cr100304n. - DOI - PubMed
- Pansare S. V. Eldho K. P. Chem.–Eur. J. 2011;17:8770. doi: 10.1002/chem.201101269. - DOI - PubMed
- Denmark S. E. Heemstra J. R. Beutner G. L. Angew. Chem., Int. Ed. 2005;44:4682. doi: 10.1002/anie.200462338. - DOI - PubMed
- Hepburn H. B. Dell’ Amico L. Melchiorre P. Chem. Rec. 2016;16:1787. doi: 10.1002/tcr.201600030. - DOI - PubMed
-
- Clayden J. Chem. Soc. Rev. 2009;38:817. doi: 10.1039/B801639A. - DOI - PubMed
- Jiang H. Albrecht Ł. Jørgensen K. A. Chem. Sci. 2013;4:2287. doi: 10.1039/C3SC50405K. - DOI
-
. For representative examples of vinylogous reactions:
- Tian X. Hofmann N. Melchiorre P. Angew. Chem., Int. Ed. 2014;53:2997. doi: 10.1002/anie.201310487. - DOI - PubMed
- Dell’ Amico L. Albrecht Ł. Naicker T. Poulsen P. H. Jørgensen K. A. J. Am. Chem. Soc. 2013;135:8063. doi: 10.1021/ja4029928. - DOI - PubMed
- Stiller J. Poulsen P. H. Cruz Cruz D. Dourado J. Davis R. L. Jørgensen K. A. Chem. Sci. 2014;5:2052. doi: 10.1039/C4SC00081A. - DOI
- Jia Z.-J. Jiang H. Li J.-L. Gschwend B. Li Q.-Z. Yin X. Grouleff J. Chen Y.-C. Jørgensen K. A. J. Am. Chem. Soc. 2011;133:5053. doi: 10.1021/ja1112194. - DOI - PubMed
- Xiong X.-F. Zhou Q. Gu J. Dong L. Liu T.-Y. Chen Y.-C. Angew. Chem., Int. Ed. 2012;51:4401. doi: 10.1002/anie.201200248. - DOI - PubMed
- Silvi M. Chatterjee I. Liu Y. Melchiorre P. Angew. Chem., Int. Ed. 2013;52:10780. doi: 10.1002/anie.201305870. - DOI - PubMed
- Bencivenni G. Galzerano P. Mazzanti A. Bartoli G. Melchiorre P. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20642. doi: 10.1073/pnas.1001150107. - DOI - PMC - PubMed
- Di Iorio N. Righi P. Mazzanti A. Mancinelli M. Ciogli A. Bencivenni G. J. Am. Chem. Soc. 2014;136:10250. doi: 10.1021/ja505610k. - DOI - PubMed
- Albrecht Ł. Dickmeiss G. Cruz Acosta F. Rodríguez-Escrich C. Davis R. L. Jørgensen K. A. J. Am. Chem. Soc. 2012;134:5053. - PubMed
- Qi L.-W. Yang Y. Gui Y.-Y. Zhang Y. Chen F. Tian F. Peng L. Wang L.-X. Org. Lett. 2014;16:6436. doi: 10.1021/ol503266q. - DOI - PubMed
-
-
For review on the electrophilic reactivity see:
- Chris V. Galliford C. V. Scheidt K. A. Angew. Chem., Int. Ed. 2007;46:8748. doi: 10.1002/anie.200701342. - DOI - PubMed
- Dalpozzo R. Bartoli G. Bencivenni G. Chem. Soc. Rev. 2012;41:7247. doi: 10.1039/C2CS35100E. - DOI - PubMed
- Dalpozzo R. Adv. Synth. Catal. 2017;359:1772. doi: 10.1002/adsc.201700361. - DOI
- Cheng D.-J. Ishihara Y. Tan B. Barbas III C. F. ACS Catal. 2014;4:743. doi: 10.1021/cs401172r. - DOI
-
. For vinylogous reactivity see:
- Rassu G. Zambrano V. Tanca R. Sartori A. Battistini L. Zanardi F. Curti C. Casiraghi G. Eur. J. Org. Chem. 2012:466. doi: 10.1002/ejoc.201101446. - DOI
- Curti C. Rassu G. Zambrano V. Pinna L. Pelosi G. Sartori A. Battistini L. Zanardi F. Casiraghi G. Angew. Chem., Int. Ed. 2012;51:6200. doi: 10.1002/anie.201202027. - DOI - PubMed
- Chen Q. Wang G. Jiang X. Xu Z. Lin L. Wang R. Org. Lett. 2014;16:1394. doi: 10.1021/ol500157b. - DOI - PubMed
- Zhong Y. Ma S. Xu Z. Changa M. Wang R. RSC Adv. 2014;4:49930. doi: 10.1039/C4RA09128K. - DOI
- Feng J. Li X. Cheng J.-P. Chem. Commun. 2015;51:14342. doi: 10.1039/C5CC06182B. - DOI - PubMed
- Di Iorio N. Righi P. Ranieri S. Mazzanti A. Margutta R. G. Bencivenni G. J. Org. Chem. 2015;80:7158. doi: 10.1021/acs.joc.5b01022. - DOI - PubMed
- Xiao X. Mei H. Chen Q. Zhao L. Lin X. Liu X. Feng X. Chem. Commun. 2015;51:580. doi: 10.1039/C4CC07204A. - DOI - PubMed
- Liu Y. Yang Y. Huang Y. Xu X.-H. Qing F.-L. Synlett. 2015;26:72.
- Feng J. Li X. Cheng J.-P. J. Org. Chem. 2017;82:1412. doi: 10.1021/acs.joc.6b02582. - DOI - PubMed
- Jadhav A. P. Ali A. Singh R. P. Adv. Synth. Catal. 2017;359:1508. doi: 10.1002/adsc.201601265. - DOI
-
-
- Petrov V. A., Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications, John Wiley & Sons, Hoboken, NJ, 2009, p. 397
- O'Hagan D. J. Fluorine Chem. 2010;131:1071. doi: 10.1016/j.jfluchem.2010.03.003. - DOI
LinkOut - more resources
Full Text Sources

