The synthesis of HMF-based α-amino phosphonates via one-pot Kabachnik-Fields reaction
- PMID: 35548197
- PMCID: PMC9085609
- DOI: 10.1039/c8ra05983g
The synthesis of HMF-based α-amino phosphonates via one-pot Kabachnik-Fields reaction
Abstract
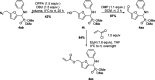
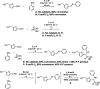
The first use of biomass-derived HMF in the one-pot Kabachnik-Fields reaction is reported here. A wide range of furan-based α-amino phosphonates were prepared in moderate to excellent yields under mild, effective and environmentally-benign conditions: iodine as a non-metal catalyst, biobased 2-MeTHF as the solvent and room or moderate temperature. The hydroxymethyl group of HMF persists in the Kabachnik-Fields products, widening the scope of further modification and derivatization compared to those arising from furfural. Issues involving the diastereoselectivity and double Kabachnik-Fields condensation were also faced.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

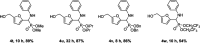

Similar articles
-
Photooxidation Coupled Kabachnik-Fields and Bigenelli reactions for Direct Conversion of Benzyl alcohols to α-Amino Phosphonates and Dihydropyrimidones.Acta Chim Slov. 2020 Mar;67(1):195-202. Acta Chim Slov. 2020. PMID: 33558909
-
Study on the Microwave-Assisted Batch and Continuous Flow Synthesis of N-Alkyl-Isoindolin-1-One-3-Phosphonates by a Special Kabachnik-Fields Condensation.Molecules. 2020 Jul 21;25(14):3307. doi: 10.3390/molecules25143307. Molecules. 2020. PMID: 32708227 Free PMC article.
-
A divergent approach for the synthesis of (hydroxymethyl)furfural (HMF) from spent aromatic biomass-derived (chloromethyl)furfural (CMF) as a renewable feedstock.RSC Adv. 2020 Dec 21;10(73):45081-45089. doi: 10.1039/d0ra09310f. eCollection 2020 Dec 17. RSC Adv. 2020. PMID: 35516261 Free PMC article.
-
The Kabachnik-Fields reaction: mechanism and synthetic use.Molecules. 2012 Nov 1;17(11):12821-35. doi: 10.3390/molecules171112821. Molecules. 2012. PMID: 23117425 Free PMC article. Review.
-
One-Pot Conversion of Carbohydrates into Furan Derivatives via Furfural and 5-Hydroxylmethylfurfural as Intermediates.ChemSusChem. 2016 Aug 23;9(16):2015-36. doi: 10.1002/cssc.201600507. Epub 2016 Jul 11. ChemSusChem. 2016. PMID: 27396713 Review.
Cited by
-
Direct Synthesis of Phosphonates and α-Amino-phosphonates from 1,3-Benzoxazines.Molecules. 2019 Jan 15;24(2):294. doi: 10.3390/molecules24020294. Molecules. 2019. PMID: 30650579 Free PMC article.
-
Nanostructured N doped TiO2 efficient stable catalyst for Kabachnik-Fields reaction under microwave irradiation.RSC Adv. 2020 Jul 20;10(45):26997-27005. doi: 10.1039/d0ra04533k. eCollection 2020 Jul 15. RSC Adv. 2020. PMID: 35515785 Free PMC article.
-
Synthesis of α-Aminophosphonates and Related Derivatives; the Last Decade of the Kabachnik-Fields Reaction.Molecules. 2021 Apr 25;26(9):2511. doi: 10.3390/molecules26092511. Molecules. 2021. PMID: 33923090 Free PMC article. Review.
-
Phosphonates enantiomers receiving with fungal enzymatic systems.Microb Cell Fact. 2021 Apr 7;20(1):81. doi: 10.1186/s12934-021-01573-8. Microb Cell Fact. 2021. PMID: 33827578 Free PMC article. Review.
References
-
- Mika L. T. Csefalvay E. Nemeth A. Chem. Rev. 2018;118:505. doi: 10.1021/acs.chemrev.7b00395. - DOI - PubMed
- Zhang Z. Song J. Han B. Chem. Rev. 2017;117:6834. doi: 10.1021/acs.chemrev.6b00457. - DOI - PubMed
- Chen S. S. Maneerung T. Tsang D. C. W. Ok Y. S. Wang C.-H. Chem. Eng. J. 2017;328:246. doi: 10.1016/j.cej.2017.07.020. - DOI
- Shylesh S. Gokhale A. A. Ho C. R. Bell A. T. Acc. Chem. Res. 2017;50:2589. doi: 10.1021/acs.accounts.7b00354. - DOI - PubMed
- de Vries J. G. Chem. Rec. 2016;16:2787. doi: 10.1002/tcr.201600102. - DOI - PubMed
- Wu L. Moteki T. Gokhale A. A. Flaherty D. W. Toste F. D. Chem. 2016;1:32. doi: 10.1016/j.chempr.2016.05.002. - DOI
- Besson M. Gallezot P. Pinel C. Chem. Rev. 2014;114:1827. doi: 10.1021/cr4002269. - DOI - PubMed
- Gallezot P. Chem. Soc. Rev. 2012;41:1538. doi: 10.1039/C1CS15147A. - DOI - PubMed
-
- de Vries J. G., in Adv. Heterocycl. Chem., ed. E. F. V. Scriven and C. A. Ramsden, Academic Press, 2017, vol. 121, p. 247
- Domínguez de María P. Guajardo N. ChemSusChem. 2017;10:4123. doi: 10.1002/cssc.201701583. - DOI - PubMed
- Hu L. Lin L. Wu Z. Zhou S. Liu S. Renewable Sustainable Energy Rev. 2017;74:230. doi: 10.1016/j.rser.2017.02.042. - DOI
- van Putten R.-J. van der Waal J. C. de Jong E. Rasrendra C. B. Heeres H. J. de Vries J. G. Chem. Rev. 2013;113:1499. doi: 10.1021/cr300182k. - DOI - PubMed
- Rosatella A. A. Simeonov S. P. Frade R. F. M. Afonso C. A. M. Green Chem. 2011;13:754. doi: 10.1039/C0GC00401D. - DOI
- Kucherov F. A. Romashov L. V. Galkin K. I. Ananikov V. P. ACS Sustainable Chem. Eng. 2018;6:8064. doi: 10.1021/acssuschemeng.8b00971. - DOI
-
- Biswas S. Dutta B. Mannodi-Kanakkithodi A. Clarke R. Song W. Ramprasad R. Suib S. L. Chem. Commun. 2017;53:11751. doi: 10.1039/C7CC06097A. - DOI - PubMed
- Gong W. Zheng K. Ji P. RSC Adv. 2017;7:34776. doi: 10.1039/C7RA05427K. - DOI
- Gui Z. Saravanamurugan S. Cao W. Schill L. Chen L. Qi Z. Riisager A. ChemistrySelect. 2017;2:6632. doi: 10.1002/slct.201701325. - DOI
- Li G. Sun Z. Yan Y. Zhang Y. Tang Y. ChemSusChem. 2017;10:494. doi: 10.1002/cssc.201601322. - DOI - PubMed
- Li J. Lv G. Lu B. Wang Y. Deng T. Hou X. Yang Y. Energy Technol. 2017;5:1429. doi: 10.1002/ente.201600715. - DOI
- Li Y.-M. Zhang X.-Y. Li N. Xu P. Lou W.-Y. Zong M.-H. ChemSusChem. 2017;10:304. doi: 10.1002/cssc.201700004. - DOI - PubMed
- McKenna S. M. Mines P. Law P. Kovacs-Schreiner K. Birmingham W. R. Turner N. J. Leimkuhler S. Carnell A. J. Green Chem. 2017;19:4660. doi: 10.1039/C7GC01696D. - DOI
- Mishra D. K. Lee H. J. Kim J. Lee H.-S. Cho J. K. Suh Y.-W. Yi Y. Kim Y. J. Green Chem. 2017;19:1619. doi: 10.1039/C7GC00027H. - DOI
- Wang Q. Hou W. Li S. Xie J. Li J. Zhou Y. Wang J. Green Chem. 2017;19:3820. doi: 10.1039/C7GC01116D. - DOI
- Xu S. Zhou P. Zhang Z. Yang C. Zhang B. Deng K. Bottle S. Zhu H. J. Am. Chem. Soc. 2017;139:14775. doi: 10.1021/jacs.7b08861. - DOI - PubMed
- Zhang H. Wu Q. Guo C. Wu Y. Wu T. ACS Sustainable Chem. Eng. 2017;5:3517. doi: 10.1021/acssuschemeng.7b00231. - DOI
- Li J. Liu J.-l. Liu H.-y. Xu G.-y. Zhang J.-j. Liu J.-x. Zhou G.-l. Li Q. Xu Z.-h. Fu Y. ChemSusChem. 2017;10:1436. doi: 10.1002/cssc.201700105. - DOI - PubMed
-
- Mohamed O. G. Khalil Z. G. Capon R. J. Org. Lett. 2018;20:377. doi: 10.1021/acs.orglett.7b03666. - DOI - PubMed
- Kumalaputri A. J. Randolph C. Otten E. Heeres H. J. Deuss P. J. ACS Sustainable Chem. Eng. 2018;6:3419. doi: 10.1021/acssuschemeng.7b03648. - DOI - PMC - PubMed
- Zhu M.-M. Tao L. Zhang Q. Dong J. Liu Y.-M. He H.-Y. Cao Y. Green Chem. 2017;19:3880. doi: 10.1039/C7GC01579H. - DOI
- Kucherov F. A. Galkin K. I. Gordeev E. G. Ananikov V. P. Green Chem. 2017;19:4858. doi: 10.1039/C7GC02211E. - DOI
- Galkin K. Kucherov F. Markov O. Egorova K. Posvyatenko A. Ananikov V. Molecules. 2017;22:2210. doi: 10.3390/molecules22122210. - DOI - PMC - PubMed
- Tšupova S. Rominger F. Rudolph M. Hashmi A. S. K. Green Chem. 2016;18:5800. doi: 10.1039/C6GC01622G. - DOI
- Sugimura H. Kikuchi M. Kato S. Sekita W. Sasaki I. Tetrahedron. 2016;72:7638. doi: 10.1016/j.tet.2016.10.026. - DOI
- Romashov L. V. Ananikov V. P. Org. Biomol. Chem. 2016;14:10593. doi: 10.1039/C6OB01731B. - DOI - PubMed
- Sowmiah S. Veiros L. F. Esperanca J. M. Rebelo L. P. Afonso C. A. Org. Lett. 2015;17:5244. doi: 10.1021/acs.orglett.5b02573. - DOI - PubMed
- Koh P. F. Loh T. P. Green Chem. 2015;17:3746. doi: 10.1039/C5GC00900F. - DOI
- Antonio J. P. M. Frade R. F. M. Santos F. M. F. Coelho J. A. S. Afonso C. A. M. Gois P. M. P. Trindade A. F. RSC Adv. 2014;4:29352. doi: 10.1039/C4RA03710C. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

