Sodium dodecylsulfate-layered double hydroxide and its use in the adsorption of 17β-estradiol in wastewater
- PMID: 35548199
- PMCID: PMC9085618
- DOI: 10.1039/c8ra05726e
Sodium dodecylsulfate-layered double hydroxide and its use in the adsorption of 17β-estradiol in wastewater
Abstract
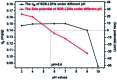
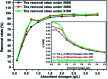
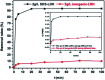
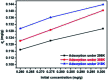
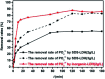
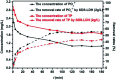
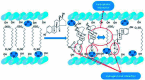
Modified Mg3Al layered double hydroxide (LDH) intercalated with dodecylsulfate anion composites, which were designated as SDS-LDH composites, were synthesized by coprecipitation. The samples were characterized using SEM, EDX, FT-IR, zeta potential analysis, and XRD. The results showed that the SDS-LDH composites contain a thicker and larger porous interconnected network than inorganic LDH due to the enlarged inter-layer distance. The outstanding adsorption performance of SDS-LDH composites toward 17β-estradiol (E2) was investigated under different conditions, including solution pH, adsorbent dosage, ion strength, reaction time, and temperature. When the solution pH was 7 and the adsorbent dosage was 2 g L-1, the removal rate of E2 reached the maximum at 94%, whereas inorganic LDH displayed a poor E2 removal rate of 10%. The presence of various ions (Na+, SO4 2-, CI-, and H2PO4 -) in aqueous solution exerted no significant adverse effects on the adsorption process. The adsorption equilibrium was reached within 20 min, and the adsorption fitted well with the pseudo-second-order model and the Freundlich isotherm. The thermodynamic test revealed that the adsorption process was spontaneous and endothermic. Phosphorus was selected as the index for evaluating the adsorption capacity of SDS-LDH composites for inorganic ions. The removal rates of total phosphorus and PO4 3- were 43.71% and 55.93% for SDS-LDH composites at 2 g L-1. The removal rate of PO4 3- reached up to 85% when the contact time was 120 min and the dosage was 3 g L-1 for SDS-LDH composites, which were approximately close to those of inorganic LDH of 30 min and 2 g L-1, respectively. This finding indicates that the removal capacity of SDS-LDH composites for PO4 3- decreased after the dodecylsulfate anions intercalated into the interlayer. The composites retained their high efficiency and stability after desorption and regeneration with alkali treatment. This study demonstrated that SDS-LDH composites are a promising adsorbent for the recovery and abatement of trace-level E2 in secondary effluents of wastewater treatment plants.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures











Similar articles
-
The enhancement roles of sulfate on the adsorption of sodium dodecylsulfate by calcium-based layered double hydroxide: microstructure and thermal behaviors.Environ Sci Pollut Res Int. 2019 Jul;26(19):19320-19326. doi: 10.1007/s11356-019-05295-8. Epub 2019 May 9. Environ Sci Pollut Res Int. 2019. PMID: 31073839
-
Mussel-inspired preparation of layered double hydroxides based polymer composites for removal of copper ions.J Colloid Interface Sci. 2019 Jan 1;533:416-427. doi: 10.1016/j.jcis.2018.08.064. Epub 2018 Aug 25. J Colloid Interface Sci. 2019. PMID: 30172152
-
Sodium dodecyl sulfate intercalated and acrylamide anchored layered double hydroxides: A multifunctional adsorbent for highly efficient removal of Congo red.J Colloid Interface Sci. 2018 Jul 1;521:172-182. doi: 10.1016/j.jcis.2018.03.040. Epub 2018 Mar 14. J Colloid Interface Sci. 2018. PMID: 29567605
-
Application of layered double hydroxide-biochar composites in wastewater treatment: Recent trends, modification strategies, and outlook.J Hazard Mater. 2021 Oct 15;420:126569. doi: 10.1016/j.jhazmat.2021.126569. Epub 2021 Jul 3. J Hazard Mater. 2021. PMID: 34280719 Review.
-
Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook.Bioresour Technol. 2021 Jan;319:124128. doi: 10.1016/j.biortech.2020.124128. Epub 2020 Sep 17. Bioresour Technol. 2021. PMID: 32979597 Review.
Cited by
-
Dielectric, Mechanical, and Thermal Properties of Crosslinked Polyethylene Nanocomposite with Hybrid Nanofillers.Polymers (Basel). 2023 Mar 29;15(7):1702. doi: 10.3390/polym15071702. Polymers (Basel). 2023. PMID: 37050316 Free PMC article.
-
Sequestration of steroidal estrogen in aqueous samples using an adsorption mechanism: a systemic scientometric review.RSC Adv. 2023 Jul 26;13(33):22675-22697. doi: 10.1039/d3ra02296j. eCollection 2023 Jul 26. RSC Adv. 2023. PMID: 37502828 Free PMC article. Review.
-
Investigation of organophosphorus (OPs) compounds by a needle trap device based on mesoporous organo-layered double hydroxide (organo-LDH).RSC Adv. 2023 Jun 12;13(26):17656-17666. doi: 10.1039/d3ra01732j. eCollection 2023 Jun 9. RSC Adv. 2023. PMID: 37312990 Free PMC article.
-
Study on Synergistically Improving Corrosion Resistance of Microarc Oxidation Coating on Magnesium Alloy by Loading of Sodium Tungstate and Silane Treatment.Materials (Basel). 2025 Jan 14;18(2):361. doi: 10.3390/ma18020361. Materials (Basel). 2025. PMID: 39859832 Free PMC article.
-
Dioctylsulfosuccinate Functionalized NiAl-Layered Double Hydroxide for Sensitive Fenuron Electroanalysis Using a Carbon Paste Electrode.J Anal Methods Chem. 2024 Jul 19;2024:9237309. doi: 10.1155/2024/9237309. eCollection 2024. J Anal Methods Chem. 2024. PMID: 39377042 Free PMC article.
References
-
- Fernandez L. Borzecka W. Lin Z. Schneider R. J. Huvaere K. Esteves V. I. Cunha A. Tome J. P. C. Nanomagnet-photosensitizer hybrid materials for the degradation of E2 in batch and flow modes. Dyes Pigm. 2017;142:535–543. doi: 10.1016/j.dyepig.2017.04.010. - DOI
-
- Caliman F. A. Gavrilescu M. Pharmaceuticals, personal care products and endocrine disrupting agents in the environment: a review. Clean. 2009;37:277–303.
-
- Wang Q. et al., Polypropylene/layered double hydroxide nanocomposites. J. Mater. Chem. 2012;22(36):19113–19121. doi: 10.1039/C2JM33493C. - DOI
LinkOut - more resources
Full Text Sources

