Acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition for the synthesis of quinolines under air
- PMID: 35548218
- PMCID: PMC9085790
- DOI: 10.1039/c8ra06826g
Acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition for the synthesis of quinolines under air
Abstract
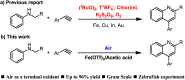
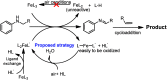
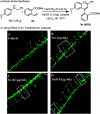
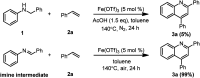
An acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition reaction was developed for the synthesis of quinolines using air as a terminal oxidant. Acetic acid was the best cocatalyst for the cycloaddition of N-alkyl anilines with alkenes or alkynes under air. Various quinoline derivatives were obtained in satisfactory-to-excellent yields, and no other byproducts besides water were produced in the reaction. The zebrafish model has become an important vertebrate model for evaluating drug effects. We tested the activity of 3n in zebrafish. The test results showed that 1 μg mL-13n treatments resulted in morphological malformation, and 0.01-0.1 μg mL-13n treatments led to potent angiogenic defects in zebrafish embryos. The results of this study will be of great significance for promoting drug research in cardiovascular and cerebrovascular diseases.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Environmentally Benign Synthesis of Quinoline-Spiroquinazolinones by Iron-Catalyzed Dehydrogenative [4 + 2] Cycloaddition of Secondary/Tertiary Anilines and 4-Methylene-quinazolinones.J Org Chem. 2021 Sep 3;86(17):12257-12266. doi: 10.1021/acs.joc.1c01602. Epub 2021 Aug 13. J Org Chem. 2021. PMID: 34387487
-
TEMPO oxoammonium salt-mediated dehydrogenative Povarov/oxidation tandem reaction of N-alkyl anilines.Org Lett. 2011 Nov 18;13(22):6066-9. doi: 10.1021/ol202552y. Epub 2011 Oct 18. Org Lett. 2011. PMID: 22007655
-
Ruthenium-catalysed synthesis of 2- and 3-substituted quinolines from anilines and 1,3-diols.Org Biomol Chem. 2011 Jan 21;9(2):610-5. doi: 10.1039/c0ob00676a. Epub 2010 Nov 22. Org Biomol Chem. 2011. PMID: 21103492
-
Synthesis of Quinolines via a Metal-Catalyzed Dehydrogenative N-Heterocyclization.Chem Rec. 2017 Feb;17(2):200-216. doi: 10.1002/tcr.201600083. Epub 2016 Aug 15. Chem Rec. 2017. PMID: 27524555 Review.
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
Cited by
-
Ligand-Promoted Iridium-Catalyzed Transfer Hydrogenation of Terminal Alkynes with Ethanol and Its Application.ACS Omega. 2019 Sep 18;4(14):16045-16051. doi: 10.1021/acsomega.9b02191. eCollection 2019 Oct 1. ACS Omega. 2019. PMID: 31592175 Free PMC article.
-
Recent synthetic efforts in the preparation of 2-(3,4)-alkenyl (aryl) quinoline molecules towards anti-kinetoplastid agents.RSC Adv. 2020 Jan 29;10(9):4876-4898. doi: 10.1039/c9ra09905k. eCollection 2020 Jan 29. RSC Adv. 2020. PMID: 35498276 Free PMC article. Review.
References
-
-
For selected reviews, see:
- Orhan Puskullu M. Tekiner B. Suzen S. Mini-Rev. Med. Chem. 2013;13:365. - PubMed
- Gopaul K. Shintre S. A. Koorbanally N. A. Anti-Cancer Agents Med. Chem. 2015;15:631. doi: 10.2174/1871520615666141216125446. - DOI - PubMed
- Afzal O. Kumar S. Haider M. R. Ali M. R. Kumar R. Jaggi M. Bawa S. Eur. J. Med. Chem. 2015;97:871. doi: 10.1016/j.ejmech.2014.07.044. - DOI - PubMed
-
-
-
For selected reviews, see:
- ElSohly M. A. Gul W. Recent Pat. Anti-Infect. Drug Discovery. 2007;2:222. doi: 10.2174/157489107782497263. - DOI - PubMed
- Zhang Y. Han T. Ming Q. Wu L. Rahman K. Qin L. Nat. Prod. Commun. 2012;7:963. - PubMed
- Chung P.-Y. Bian Z.-X. Pun H.-Y. Chan D. Chan A. S.-C. Chui C.-H. Tang J. C.-O. Lam K.-H. Future Med. Chem. 2015;7:947. doi: 10.4155/fmc.15.34. - DOI - PubMed
-
-
-
For related reviews, see:
- Marco-Contelles J. Pérez-Mayoral E. Samadi A. Carreiras M. d. C. Soriano E. Chem. Rev. 2009;109:2652. doi: 10.1021/cr800482c. - DOI - PubMed
- Majumder A. Gupta R. Jain A. Green Chem. Lett. Rev. 2013;6:151. doi: 10.1080/17518253.2012.733032. - DOI
- Prajapati S. M. Patel K. D. Vekariya R. H. Panchal S. N. Patel H. D. RSC Adv. 2014;4:24463. doi: 10.1039/C4RA01814A. - DOI
- Khusnutdinov R. I. Bayguzina A. R. Dzhemilev U. M. J. Organomet. Chem. 2014;768:75. doi: 10.1016/j.jorganchem.2014.06.008. - DOI
- Eftekhari-Sis B. Zirak M. Chem. Rev. 2015;115:151. doi: 10.1021/cr5004216. - DOI - PubMed
- Ramann G. A. Cowen B. J. Molecules. 2016;21:986. doi: 10.3390/molecules21080986. - DOI - PMC - PubMed
- Batista V. F. Pinto D. C. G. A. Silva A. M. S. ACS Sustainable Chem. Eng. 2016;4:4064. doi: 10.1021/acssuschemeng.6b01010. - DOI
- Chelucci G. Porcheddu A. Chem. Rec. 2017;17:200. doi: 10.1002/tcr.201600083. - DOI - PubMed
-
-
- Richter H. Mancheño O. G. Org. Lett. 2011;13:6066. doi: 10.1021/ol202552y. - DOI - PubMed
- Liu P. Wang Z. Lin J. Hu X. Eur. J. Org. Chem. 2012:1583. doi: 10.1002/ejoc.201101656. - DOI
- Liu P. Li Y. Wang H. Wang Z. Hu X. Tetrahedron Lett. 2012;53:6654. doi: 10.1016/j.tetlet.2012.09.090. - DOI
- Rohlmann R. Stopka T. Richter H. Mancheño O. G. J. Org. Chem. 2013;78:6050. doi: 10.1021/jo4007199. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

