Acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition for the synthesis of quinolines under air
- PMID: 35548218
- PMCID: PMC9085790
- DOI: 10.1039/c8ra06826g
Acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition for the synthesis of quinolines under air
Abstract
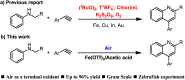
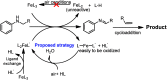
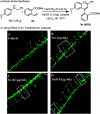
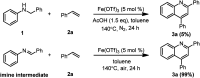
An acid-promoted iron-catalysed dehydrogenative [4 + 2] cycloaddition reaction was developed for the synthesis of quinolines using air as a terminal oxidant. Acetic acid was the best cocatalyst for the cycloaddition of N-alkyl anilines with alkenes or alkynes under air. Various quinoline derivatives were obtained in satisfactory-to-excellent yields, and no other byproducts besides water were produced in the reaction. The zebrafish model has become an important vertebrate model for evaluating drug effects. We tested the activity of 3n in zebrafish. The test results showed that 1 μg mL-13n treatments resulted in morphological malformation, and 0.01-0.1 μg mL-13n treatments led to potent angiogenic defects in zebrafish embryos. The results of this study will be of great significance for promoting drug research in cardiovascular and cerebrovascular diseases.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
-
For selected reviews, see:
- Orhan Puskullu M. Tekiner B. Suzen S. Mini-Rev. Med. Chem. 2013;13:365. - PubMed
- Gopaul K. Shintre S. A. Koorbanally N. A. Anti-Cancer Agents Med. Chem. 2015;15:631. doi: 10.2174/1871520615666141216125446. - DOI - PubMed
- Afzal O. Kumar S. Haider M. R. Ali M. R. Kumar R. Jaggi M. Bawa S. Eur. J. Med. Chem. 2015;97:871. doi: 10.1016/j.ejmech.2014.07.044. - DOI - PubMed
-
-
-
For selected reviews, see:
- ElSohly M. A. Gul W. Recent Pat. Anti-Infect. Drug Discovery. 2007;2:222. doi: 10.2174/157489107782497263. - DOI - PubMed
- Zhang Y. Han T. Ming Q. Wu L. Rahman K. Qin L. Nat. Prod. Commun. 2012;7:963. - PubMed
- Chung P.-Y. Bian Z.-X. Pun H.-Y. Chan D. Chan A. S.-C. Chui C.-H. Tang J. C.-O. Lam K.-H. Future Med. Chem. 2015;7:947. doi: 10.4155/fmc.15.34. - DOI - PubMed
-
-
-
For related reviews, see:
- Marco-Contelles J. Pérez-Mayoral E. Samadi A. Carreiras M. d. C. Soriano E. Chem. Rev. 2009;109:2652. doi: 10.1021/cr800482c. - DOI - PubMed
- Majumder A. Gupta R. Jain A. Green Chem. Lett. Rev. 2013;6:151. doi: 10.1080/17518253.2012.733032. - DOI
- Prajapati S. M. Patel K. D. Vekariya R. H. Panchal S. N. Patel H. D. RSC Adv. 2014;4:24463. doi: 10.1039/C4RA01814A. - DOI
- Khusnutdinov R. I. Bayguzina A. R. Dzhemilev U. M. J. Organomet. Chem. 2014;768:75. doi: 10.1016/j.jorganchem.2014.06.008. - DOI
- Eftekhari-Sis B. Zirak M. Chem. Rev. 2015;115:151. doi: 10.1021/cr5004216. - DOI - PubMed
- Ramann G. A. Cowen B. J. Molecules. 2016;21:986. doi: 10.3390/molecules21080986. - DOI - PMC - PubMed
- Batista V. F. Pinto D. C. G. A. Silva A. M. S. ACS Sustainable Chem. Eng. 2016;4:4064. doi: 10.1021/acssuschemeng.6b01010. - DOI
- Chelucci G. Porcheddu A. Chem. Rec. 2017;17:200. doi: 10.1002/tcr.201600083. - DOI - PubMed
-
-
- Richter H. Mancheño O. G. Org. Lett. 2011;13:6066. doi: 10.1021/ol202552y. - DOI - PubMed
- Liu P. Wang Z. Lin J. Hu X. Eur. J. Org. Chem. 2012:1583. doi: 10.1002/ejoc.201101656. - DOI
- Liu P. Li Y. Wang H. Wang Z. Hu X. Tetrahedron Lett. 2012;53:6654. doi: 10.1016/j.tetlet.2012.09.090. - DOI
- Rohlmann R. Stopka T. Richter H. Mancheño O. G. J. Org. Chem. 2013;78:6050. doi: 10.1021/jo4007199. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

