Sulfur(vi) fluoride exchange as a key reaction for synthesizing biaryl sulfate core derivatives as potent hepatitis C virus NS5A inhibitors and their structure-activity relationship studies
- PMID: 35548241
- PMCID: PMC9085918
- DOI: 10.1039/c8ra05471a
Sulfur(vi) fluoride exchange as a key reaction for synthesizing biaryl sulfate core derivatives as potent hepatitis C virus NS5A inhibitors and their structure-activity relationship studies
Abstract
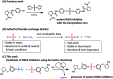
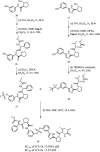
Extremely potent, new hepatitis C virus (HCV) nonstructural 5A (NS5A) featuring substituted biaryl sulfate core structures was designed and synthesized. Based on the previously reported novel HCV NS5A inhibitors featuring biaryl sulfate core structures which exhibit two-digit picomolar half-maximal effective concentration (EC50) values against HCV genotype 1b and 2a, the new inhibitors equipped with the sulfate core structures containing diversely substituted aryl groups were explored. In this study, highly efficient, chemoselective coupling reactions between an arylsulfonyl fluoride and an aryl silyl ether, known as the sulfur(vi) fluoride exchange (SuFEx) reaction, were utilized. Among the inhibitors prepared based on the SuFEx chemistry, compounds 14, 15 and 29 exhibited two-digit picomolar EC50 values against GT-1b and single digit or sub nanomolar activities against the HCV GT-2a strain. Nonsymmetrical inhibitors containing an imidazole and amide moieties on each side of the sulfate core structures were also synthesized. In addition, a biotinylated probe targeting NS5A protein was prepared for labeling using the same synthetic methodology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare regarding this study.
Figures
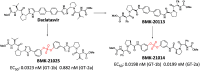
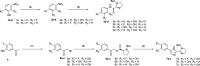

Similar articles
-
New potent biaryl sulfate-based hepatitis C virus inhibitors.Eur J Med Chem. 2017 Jan 5;125:87-100. doi: 10.1016/j.ejmech.2016.09.031. Epub 2016 Sep 10. Eur J Med Chem. 2017. PMID: 27657807
-
Design and Synthesis of Novel Bis-Imidazolyl Phenyl Butadiyne Derivatives as HCV NS5A Inhibitors.Pharmaceuticals (Basel). 2022 May 20;15(5):632. doi: 10.3390/ph15050632. Pharmaceuticals (Basel). 2022. PMID: 35631457 Free PMC article.
-
Redesigning of the cap conformation and symmetry of the diphenylethyne core to yield highly potent pan-genotypic NS5A inhibitors with high potency and high resistance barrier.Eur J Med Chem. 2022 Feb 5;229:114034. doi: 10.1016/j.ejmech.2021.114034. Epub 2021 Dec 7. Eur J Med Chem. 2022. PMID: 34959173
-
Symmetric Anti-HCV Agents: Synthesis, Antiviral Properties, and Conformational Aspects of Core Scaffolds.ACS Omega. 2019 Jul 1;4(7):11440-11454. doi: 10.1021/acsomega.9b01242. eCollection 2019 Jul 31. ACS Omega. 2019. PMID: 31460249 Free PMC article.
-
The non-structural 5A protein of hepatitis C virus.J Viral Hepat. 1999 Sep;6(5):343-56. doi: 10.1046/j.1365-2893.1999.00185.x. J Viral Hepat. 1999. PMID: 10607250 Review.
Cited by
-
Sulfur fluoride exchange.Nat Rev Methods Primers. 2023;3:58. Epub 2023 Aug 3. Nat Rev Methods Primers. 2023. PMID: 38873592 Free PMC article.
-
Sulfur(VI) Fluoride Exchange (SuFEx)-Mediated Synthesis of the Chitosan-PEG Conjugate and Its Supramolecular Hydrogels for Protein Delivery.Nanomaterials (Basel). 2021 Jan 27;11(2):318. doi: 10.3390/nano11020318. Nanomaterials (Basel). 2021. PMID: 33513757 Free PMC article.
-
Sulfur(VI) Fluoride Exchange Chemistry in Solid-Phase Synthesis of Compound Arrays: Discovery of Histone Deacetylase Inhibitors.JACS Au. 2024 Apr 17;4(5):1854-1862. doi: 10.1021/jacsau.4c00042. eCollection 2024 May 27. JACS Au. 2024. PMID: 38818074 Free PMC article.
-
Click Chemistry for Biofunctional Polymers: From Observing to Steering Cell Behavior.Chem Rev. 2024 Dec 11;124(23):13216-13300. doi: 10.1021/acs.chemrev.4c00251. Epub 2024 Dec 2. Chem Rev. 2024. PMID: 39621547 Free PMC article. Review.
-
New strategies to enhance the efficiency and precision of drug discovery.Front Pharmacol. 2025 Feb 11;16:1550158. doi: 10.3389/fphar.2025.1550158. eCollection 2025. Front Pharmacol. 2025. PMID: 40008135 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

