Alpinumisoflavone attenuates lipopolysaccharide-induced acute lung injury by regulating the effects of anti-oxidation and anti-inflammation both in vitro and in vivo
- PMID: 35548248
- PMCID: PMC9085634
- DOI: 10.1039/c8ra04098b
Alpinumisoflavone attenuates lipopolysaccharide-induced acute lung injury by regulating the effects of anti-oxidation and anti-inflammation both in vitro and in vivo
Abstract
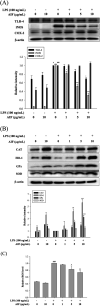
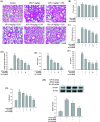
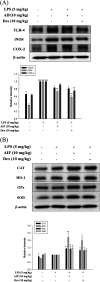
Alpinumisoflavone (AIF) is a plant-derived pyranoisoflavone that exhibits a number of pharmacological activities, but the protective effects of AIF against pulmonary inflammation are still unknown. This study aimed to investigate the anti-inflammatory effects and possible molecular mechanisms of AIF in both lipopolysaccharide (LPS)-stimulated macrophages and mice. The results revealed that AIF dramatically suppressed the production of pro-inflammatory mediators [including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, IL-17, intercellular adhesion molecule-1 (ICAM-1), and nitric oxide (NO)] and increased the levels of anti-oxidative enzymes [including catalase (CAT), heme oxygenase-1 (HO-1), glutathione peroxidase (GPx), and superoxide dismutase (SOD)] both in vitro and in vivo. Additionally, pre-treatment with AIF could not only significantly prevent histopathological changes and neutrophil infiltration but also decreased the expression levels of nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and the nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome, as well as IL-17 production in LPS-induced lung tissues. The anti-inflammatory effects of AIF were mediated by up-regulating anti-oxidative enzymes and suppressing the NF-κB, MAPK, NLRP3 inflammasome and IL-17 signaling pathways. This is the first study to reveal that AIF has a protective effect against LPS-induced lung injury in mice.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Isorhynchophylline exerts anti-inflammatory and anti-oxidative activities in LPS-stimulated murine alveolar macrophages.Life Sci. 2019 Apr 15;223:137-145. doi: 10.1016/j.lfs.2019.03.017. Epub 2019 Mar 8. Life Sci. 2019. PMID: 30858121
-
Anti-inflammatory activity of Anchusa italica Retz. in LPS-stimulated RAW264.7 cells mediated by the Nrf2/HO-1, MAPK and NF-κB signaling pathways.J Ethnopharmacol. 2022 Mar 25;286:114899. doi: 10.1016/j.jep.2021.114899. Epub 2021 Dec 6. J Ethnopharmacol. 2022. PMID: 34883218
-
S-allylmercaptocysteine ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammation and oxidative stress via nuclear factor kappa B and Keap1/Nrf2 pathways.Int Immunopharmacol. 2020 Apr;81:106273. doi: 10.1016/j.intimp.2020.106273. Epub 2020 Mar 5. Int Immunopharmacol. 2020. PMID: 32070920
-
Phloretin attenuates LPS-induced acute lung injury in mice via modulation of the NF-κB and MAPK pathways.Int Immunopharmacol. 2016 Nov;40:98-105. doi: 10.1016/j.intimp.2016.08.035. Epub 2016 Aug 30. Int Immunopharmacol. 2016. PMID: 27588909
-
A Pharmacological Overview of Alpinumisoflavone, a Natural Prenylated Isoflavonoid.Front Pharmacol. 2019 Sep 10;10:952. doi: 10.3389/fphar.2019.00952. eCollection 2019. Front Pharmacol. 2019. PMID: 31551770 Free PMC article. Review.
Cited by
-
Alpinumisoflavone ameliorates choroidal neovascularisation and fibrosis in age-related macular degeneration in in vitro and in vivo models.Sci Rep. 2022 Aug 22;12(1):14316. doi: 10.1038/s41598-022-18531-y. Sci Rep. 2022. PMID: 35995845 Free PMC article.
-
Astringin protects LPS-induced toxicity by suppressing oxidative stress and inflammation via suppression of PI3K/AKT/NF-κB pathway for pediatric acute lung injury.Naunyn Schmiedebergs Arch Pharmacol. 2023 Oct;396(10):2369-2377. doi: 10.1007/s00210-023-02439-z. Epub 2023 May 17. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37193771
-
Four New Iridoid Metabolites Have Been Isolated from the Stems of Neonauclea reticulata (Havil.) Merr. with Anti-Inflammatory Activities on LPS-Induced RAW264.7 Cells.Molecules. 2019 Nov 23;24(23):4271. doi: 10.3390/molecules24234271. Molecules. 2019. PMID: 31771186 Free PMC article.
-
Protective effect of Tao Hong Si Wu Decoction against inflammatory injury caused by intestinal flora disorders in an ischemic stroke mouse model.BMC Complement Med Ther. 2024 Mar 18;24(1):124. doi: 10.1186/s12906-024-04417-1. BMC Complement Med Ther. 2024. PMID: 38500092 Free PMC article.
-
The potential mechanism of huazhuojiedu decoction in the treatment of ulcerative colitis based on network pharmacology and experimental validation.Front Pharmacol. 2022 Oct 14;13:1033874. doi: 10.3389/fphar.2022.1033874. eCollection 2022. Front Pharmacol. 2022. PMID: 36313293 Free PMC article.
References
-
- Baskar N. Devi B. P. Kumar R. M. Anti-cancer activity of methanol extract of root bark of Erythrina variegata Linn. International Journal of Toxicological and Pharmacological Research. 2010;2:74–76.
-
- Namkoong S. Kim T. J. Jang I. S. Kang K. W. Oh W. K. Park J. Alpinumisoflavone induces apoptosis and suppresses extracellular signal-regulated kinases/mitogen activated protein kinase and nuclear factor-κB pathways in lung tumor cells. Biol. Pharm. Bull. 2011;34:203–208. doi: 10.1248/bpb.34.203. - DOI - PubMed
-
- Kingsford-Adaboh R. Dittrich B. Hübschle C. B. Gbewonyo W. S. Okamoto H. Kimura M. Ishida H. Invariom structure refinement, electrostatic potential and toxicity of 4-O-methylalpinumisoflavone, O,O-dimethylalpinumisoflavone and 5-O-methyl-4-O-(3-methylbut-2-en-1-yl)alpinumisoflavone. Acta Crystallogr., Sect. B: Struct. Sci. 2006;62:843–849. doi: 10.1107/S0108768106019616. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

