The molecular neurobiology and neuropathology of opioid use disorder
- PMID: 35548327
- PMCID: PMC9090195
- DOI: 10.1016/j.crneur.2021.100023
The molecular neurobiology and neuropathology of opioid use disorder
Abstract
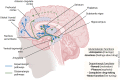
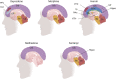
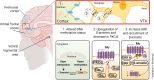
The number of people diagnosed with opioid use disorder has skyrocketed as a consequence of the opioid epidemic and the increased prescribing of opioid drugs for chronic pain relief. Opioid use disorder is characterized by loss of control of drug taking, continued drug use in the presence of adverse consequences, and repeated relapses to drug taking even after long periods of abstinence. Patients who suffer from opioid use disorder often present with cognitive deficits that are potentially secondary to structural brain abnormalities that vary according to the chemical composition of the abused opioid. This review details the neurobiological effects of oxycodone, morphine, heroin, methadone, and fentanyl on brain neurocircuitries by presenting the acute and chronic effects of these drugs on the human brain. In addition, we review results of neuroimaging in opioid use disorder patients and/or histological studies from brains of patients who had expired after acute intoxication following long-term use of these drugs. Moreover, we include relevant discussions of the neurobiological mechanisms involved in promoting abnormalities in the brains of opioid-exposed patients. Finally, we discuss how novel strategies could be used to provide pharmacological treatment against opioid use disorder.
Keywords: Fentanyl; Heroin; Methadone; Neuroimaging; Oxycodone; Postmortem.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
NP Safe Prescribing of Controlled Substances While Avoiding Drug Diversion.2023 Jan 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Jan 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 33232099 Free Books & Documents.
-
Florida Controlled Substance Prescribing.2022 Oct 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2022 Oct 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 33428370 Free Books & Documents.
-
Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone).Pain Pract. 2008 Jul-Aug;8(4):287-313. doi: 10.1111/j.1533-2500.2008.00204.x. Epub 2008 May 23. Pain Pract. 2008. PMID: 18503626
-
Opioids, Opioid Antagonists.2020 Nov 24. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2020 Nov 24. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31643200 Free Books & Documents. Review.
-
Respiratory depression and brain hypoxia induced by opioid drugs: Morphine, oxycodone, heroin, and fentanyl.Neuropharmacology. 2019 Jun;151:219-226. doi: 10.1016/j.neuropharm.2019.02.008. Epub 2019 Feb 5. Neuropharmacology. 2019. PMID: 30735692 Free PMC article. Review.
Cited by
-
Association between combined use of epidural analgesia and oxytocin administration during labor and offspring outcomes: a narrative review and proposal.Nagoya J Med Sci. 2024 Nov;86(4):549-563. doi: 10.18999/nagjms.86.4.549. Nagoya J Med Sci. 2024. PMID: 39780929 Free PMC article. Review.
-
Escalated Oxycodone Self-Administration Is Associated with Activation of Specific Gene Networks in the Rat Dorsal Striatum.Int J Mol Sci. 2025 Jul 30;26(15):7356. doi: 10.3390/ijms26157356. Int J Mol Sci. 2025. PMID: 40806484 Free PMC article.
-
Predicting postoperative chronic opioid use with fair machine learning models integrating multi-modal data sources: a demonstration of ethical machine learning in healthcare.J Am Med Inform Assoc. 2025 Jun 1;32(6):985-997. doi: 10.1093/jamia/ocaf053. J Am Med Inform Assoc. 2025. PMID: 40152140
-
Escalated oxycodone self-administration is associated with expression of voltage gated and calcium activated potassium channels in the mesocorticolimbic system in rats.Front Pharmacol. 2025 Aug 11;16:1653356. doi: 10.3389/fphar.2025.1653356. eCollection 2025. Front Pharmacol. 2025. PMID: 40860865 Free PMC article.
-
Epigenetic and Genetic Factors Associated With Opioid Use Disorder: Are These Relevant to African American Populations.Front Pharmacol. 2021 Dec 22;12:798362. doi: 10.3389/fphar.2021.798362. eCollection 2021. Front Pharmacol. 2021. PMID: 35002733 Free PMC article. Review.
References
-
- Adamides A.A., Cooper D.J., Rosenfeldt F.L., Bailey M.J., Pratt N., Tippett N.…Rosenfeld J.V. Focal cerebral oxygenation and neurological outcome with or without brain tissue oxygen-guided therapy in patients with traumatic brain injury. Acta Neurochir. 2009;151(11):1399–1409. doi: 10.1007/s00701-009-0398-y. - DOI - PubMed
-
- Bach P., Frischknecht U., Reinhard I., Bekier N., Demirakca T., Ende G.…Hermann D. Impaired working memory performance in opioid-dependent patients is related to reduced insula gray matter volume: a voxel-based morphometric study. Eur. Arch. Psychiatr. Clin. Neurosci. 2019 doi: 10.1007/s00406-019-01052-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

