Tissue-Protective Mechanisms of Bioactive Phytochemicals in Flap Surgery
- PMID: 35548348
- PMCID: PMC9081973
- DOI: 10.3389/fphar.2022.864351
Tissue-Protective Mechanisms of Bioactive Phytochemicals in Flap Surgery
Abstract
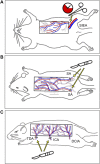
Despite careful preoperative planning, surgical flaps are prone to ischemic tissue damage and ischemia-reperfusion injury. The resulting wound breakdown and flap necrosis increase both treatment costs and patient morbidity. Hence, there is a need for strategies to promote flap survival and prevent ischemia-induced tissue damage. Phytochemicals, defined as non-essential, bioactive, and plant-derived molecules, are attractive candidates for perioperative treatment as they have little to no side effects and are well tolerated by most patients. Furthermore, they have been shown to exert beneficial combinations of pro-angiogenic, anti-inflammatory, anti-oxidant, and anti-apoptotic effects. This review provides an overview of bioactive phytochemicals that have been used to increase flap survival in preclinical animal models and discusses the underlying molecular and cellular mechanisms.
Keywords: flap; herbal medicine; ischemia–reperfusion injury; necrosis; nutraceuticals; phytochemicals.
Copyright © 2022 Weinzierl, Ampofo, Menger and Laschke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

