Hydrogen Peroxide Scavenging Restores N-Type Calcium Channels in Cardiac Vagal Postganglionic Neurons and Mitigates Myocardial Infarction-Evoked Ventricular Arrhythmias in Type 2 Diabetes Mellitus
- PMID: 35548411
- PMCID: PMC9082497
- DOI: 10.3389/fcvm.2022.871852
Hydrogen Peroxide Scavenging Restores N-Type Calcium Channels in Cardiac Vagal Postganglionic Neurons and Mitigates Myocardial Infarction-Evoked Ventricular Arrhythmias in Type 2 Diabetes Mellitus
Abstract
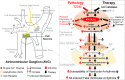
Objective: Withdrawal of cardiac vagal activity is associated with ventricular arrhythmia-related high mortality in patients with type 2 diabetes mellitus (T2DM). Our recent study found that reduced cell excitability of cardiac vagal postganglionic (CVP) neurons is involved in cardiac vagal dysfunction and further exacerbates myocardial infarction (MI)-evoked ventricular arrhythmias and mortality in T2DM. However, the mechanisms responsible for T2DM-impaired cell excitability of CVP neurons remain unclear. This study tested if and how elevation of hydrogen peroxide (H2O2) inactivates CVP neurons and contributes to cardiac vagal dysfunction and ventricular arrhythmogenesis in T2DM.
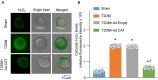
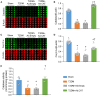
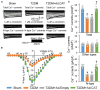
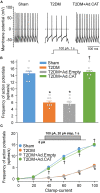
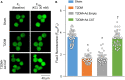
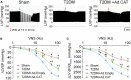
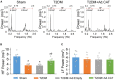
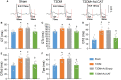
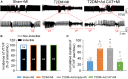
Methods and results: Rat T2DM was induced by a high-fat diet plus streptozotocin injection. Local in vivo transfection of adenoviral catalase gene (Ad.CAT) successfully induced overexpression of catalase and subsequently reduced cytosolic H2O2 levels in CVP neurons in T2DM rats. Ad.CAT restored protein expression and ion currents of N-type Ca2+ channels and increased cell excitability of CVP neurons in T2DM. Ad.CAT normalized T2DM-impaired cardiac vagal activation, vagal control of ventricular function, and heterogeneity of ventricular electrical activity. Additionally, Ad.CAT not only reduced the susceptibility to ventricular arrhythmias, but also suppressed MI-evoked lethal ventricular arrhythmias such as VT/VF in T2DM.
Conclusions: We concluded that endogenous H2O2 elevation inhibited protein expression and activation of N-type Ca2+ channels and reduced cell excitability of CVP neurons, which further contributed to the withdrawal of cardiac vagal activity and ventricular arrhythmogenesis in T2DM. Our current study suggests that the H2O2-N-type Ca2+ channel signaling axis might be an effective therapeutic target to suppress ventricular arrhythmias in T2DM patients with MI.
Keywords: calcium channel; cardiac vagal neuron; hydrogen peroxide; myocardial infarction; type 2 diabetes; ventricular arrhythmia.
Copyright © 2022 Zhang, Tu, Hu, Duan, Zimmerman and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(Th) edition. Diabetes Res Clin Pract. (2019) 157:107843. 10.1016/j.diabres.2019.107843 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

