Adaptive Immune Response to Mycobacterium abscessus Complex (MABSC) in Cystic Fibrosis and the Implications of Cross-Reactivity
- PMID: 35548464
- PMCID: PMC9084186
- DOI: 10.3389/fcimb.2022.858398
Adaptive Immune Response to Mycobacterium abscessus Complex (MABSC) in Cystic Fibrosis and the Implications of Cross-Reactivity
Abstract
Background: We aimed to characterise the adaptive immune response to Mycobacterium abscessus complex (MABSC) and its cross-reactivity with Mycobacterium avium complex (MAC) and Mycobacterium bovis (Bacille Calmette-Guérin, BCG) in cystic fibrosis (CF) patients and non-CF controls in terms of lymphocyte proliferation and immunophenotyping, cytokine production and anti-MABSC IgG plasma levels.
Methods: In this cross-sectional analysis, peripheral blood mononuclear cells (PBMC) from CF patients with MABSC (CF/MABSC, n=12), MAC infection history (CF/MAC, n=5), no NTM history (CF/NTM-, n=15), BCG-vaccinated (C/BCG+, n=9) and non-vaccinated controls (C/BCG-, n=8) were cultured for four days under stimulation with an in-house MABSC lysate and we used flow cytometry to assess lymphocyte proliferation (given by lymphoblast formation) and immunophenotypes. Cytokine production was assessed after overnight whole blood stimulation with the same lysate, and anti-MABSC IgG levels were measured in plasma from non-stimulated blood.
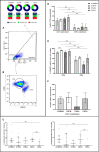
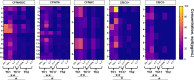
Results: All CF/MABSC patients had increased CD3+ and CD19+ lymphoblast formation upon PBMC stimulation with MABSC lysate. There was a higher rate of CD3+ than CD19+ lymphoblasts, predominance of CD4+ over CD8+ lymphoblasts, IFN-γ, TNF-α and IL-2 production, low production of the Th17-associated IL-17, and discrete or no production of Th2/B cell-associated cytokines soluble CD40 ligand (CD40L), IL-4 and IL-5, indicating a Th1-dominated phenotype and infection restricted to the lungs. A similar pattern was seen in C/BCG+ controls, and CF/MAC patients, pointing to cross-reactivity. MABSC-IgG levels were higher in CF/MABSC patients than in both control groups, but not CF/NTM- patients, most of whom also had CD3+ and/or CD19+ lymphoblast formation upon PBMC stimulation, indicating previous exposure, subclinical or latent infection with MABSC or other NTM.
Conclusion: The anti-MABSC immune response is Th1-skewed and underlines the cross-reactivity in the anti-mycobacterial immune response. The results, together with published clinical observations, indicate that BCG vaccination may cross-react against NTM in CF patients, and this should be investigated. Due to cross-reactivity, it would also be interesting to investigate whether a combination of MABSC-induced cytokine production by blood cells and anti-MABSC IgG measurement can be useful for identifying latent or subclinical infection both with MABSC and other NTM in CF patients.
Keywords: Mycobacterium abscessus Complex; Nontuberculous Mycobacteria; cellular immunology; cystic fibrosis; cytokines; flow cytometry.
Copyright © 2022 Mauch, Jensen, Qvist, Kolpen, Moser, Pressler, Nolasco da Silva, Høiby and The Copenhagen Study Group on Mycobacterial Infections in Cystic Fibrosis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

