Pseudomonas aeruginosa Infection Modulates the Immune Response and Increases Mice Resistance to Cryptococcus gattii
- PMID: 35548467
- PMCID: PMC9083911
- DOI: 10.3389/fcimb.2022.811474
Pseudomonas aeruginosa Infection Modulates the Immune Response and Increases Mice Resistance to Cryptococcus gattii
Abstract
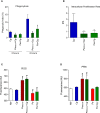
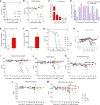
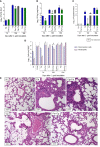
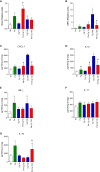
Cryptococcosis is an invasive mycosis caused by Cryptococcus spp. that affects the lungs and the central nervous system (CNS). Due to the severity of the disease, it may occur concomitantly with other pathogens, as a coinfection. Pseudomonas aeruginosa (Pa), an opportunistic pathogen, can also cause pneumonia. In this work, we studied the interaction of C. gattii (Cg) and Pa, both in vitro and in vivo. Pa reduced growth of Cg by the secretion of inhibitory molecules in vitro. Macrophages previously stimulated with Pa presented increased fungicidal activity. In vivo, previous Pa infection reduced morbidity and delayed the lethality due to cryptococcosis. This phenotype was correlated with the decreased fungal burden in the lungs and brain, showing a delay of Cg translocation to the CNS. Also, there was increased production of IL-1β, CXCL-1, and IL-10, together with the influx of iNOS-positive macrophages and neutrophils to the lungs. Altogether, Pa turned the lung into a hostile environment to the growth of a secondary pathogen, making it difficult for the fungus to translocate to the CNS. Further, iNOS inhibition reverted the Pa protective phenotype, suggesting its important role in the coinfection. Altogether, the primary Pa infection leads to balanced pro-inflammatory and anti-inflammatory responses during Cg infection. This response provided better control of cryptococcosis and was decisive for the mild evolution of the disease and prolonged survival of coinfected mice in a mechanism dependent on iNOS.
Keywords: Cryptococcosis; Cryptococcus gattii; Pseudomonas aeruginosa; coinfection; iNOS.
Copyright © 2022 Peres-Emidio, Freitas, Costa, Gouveia-Eufrasio, Silva, Santos, Carmo, Brito, Arifa, Bastos, Ribeiro, Oliveira, Silva, Paixão, Saliba, Fagundes, Souza and Santos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

