Crude Polysaccharide Extracted From Moringa oleifera Leaves Prevents Obesity in Association With Modulating Gut Microbiota in High-Fat Diet-Fed Mice
- PMID: 35548566
- PMCID: PMC9083904
- DOI: 10.3389/fnut.2022.861588
Crude Polysaccharide Extracted From Moringa oleifera Leaves Prevents Obesity in Association With Modulating Gut Microbiota in High-Fat Diet-Fed Mice
Abstract
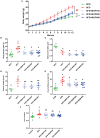
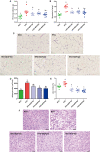
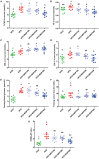
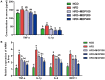
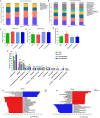
Moringa oleifera is a commonly used plant with high nutritional and medicinal values. M. oleifera leaves are considered a new food resource in China. However, the biological activities of M. oleifera polysaccharides (MOP) in regulating gut microbiota and alleviating obesity remain obscure. In the present study, we prepared the MOP and evaluated its effects on obesity and gut microbiota in high-fat diet (HFD)-induced C57BL/6J mice. The experimental mice were supplemented with a normal chow diet (NCD group), a high-fat diet (HFD group), and HFD along with MOP at a different dose of 100, 200, and 400 mg/kg/d, respectively. Physiological, histological, biochemical parameters, genes related to lipid metabolism, and gut microbiota composition were compared among five experimental groups. The results showed that MOP supplementation effectively prevented weight gain and lipid accumulation induced by HFD, ameliorated blood lipid levels and insulin resistance, alleviated the secretion of pro-inflammatory cytokines, and regulated the expression of genes related to lipid metabolism and bile acid metabolism. In addition, MOP positively reshaped the gut microbiota composition, significantly increasing the abundance of Bacteroides, norank_f_Ruminococcaceae, and Oscillibacter, while decreasing the relative abundance of Blautia, Alistipes, and Tyzzerella, which are closely associated with obesity. These results demonstrated that MOP supplementation has a protective effect against HFD-induced obesity in mice, which was associated with reshaping the gut microbiota. To the best of our knowledge, this is the first report on the potential of MOP to prevent obesity and modulating gut microbiota, which suggests that MOP can be used as a potential prebiotic.
Keywords: Moringa oleifera leaves; gut microbiota; high-fat diet; obesity; polysaccharide.
Copyright © 2022 Li, Ma, Wen, Xie, Yan, Ji, Zeng, Tian and Sheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
In vitro digestibility and prebiotic activities of a bioactive polysaccharide from Moringa oleifera leaves.J Food Biochem. 2021 Nov;45(11):e13944. doi: 10.1111/jfbc.13944. Epub 2021 Oct 13. J Food Biochem. 2021. PMID: 34642951
-
Fubrick tea attenuates high-fat diet induced fat deposition and metabolic disorder by regulating gut microbiota and caffeine metabolism.Food Funct. 2020 Aug 1;11(8):6971-6986. doi: 10.1039/d0fo01282c. Epub 2020 Jul 22. Food Funct. 2020. PMID: 32697259
-
Whole mung bean (Vigna radiata L.) supplementation prevents high-fat diet-induced obesity and disorders in a lipid profile and modulates gut microbiota in mice.Eur J Nutr. 2020 Dec;59(8):3617-3634. doi: 10.1007/s00394-020-02196-2. Epub 2020 Feb 11. Eur J Nutr. 2020. PMID: 32048004
-
The Interaction between Mushroom Polysaccharides and Gut Microbiota and Their Effect on Human Health: A Review.Biology (Basel). 2023 Jan 12;12(1):122. doi: 10.3390/biology12010122. Biology (Basel). 2023. PMID: 36671814 Free PMC article. Review.
-
The potential role of phenolic compounds on modulating gut microbiota in obesity.J Food Drug Anal. 2020 Jun 15;28(2):195-205. doi: 10.38212/2224-6614.1054. J Food Drug Anal. 2020. PMID: 35696110 Free PMC article. Review.
Cited by
-
Termite Fungus Comb Polysaccharides Alleviate Hyperglycemia and Hyperlipidemia in Type 2 Diabetic Mice by Regulating Hepatic Glucose/Lipid Metabolism and the Gut Microbiota.Int J Mol Sci. 2024 Jul 6;25(13):7430. doi: 10.3390/ijms25137430. Int J Mol Sci. 2024. PMID: 39000541 Free PMC article.
-
Ultrasonic-Cellulase Synergistic Extraction of Crude Polysaccharides from Moringa oleifera Leaves and Alleviation of Insulin Resistance in HepG2 Cells.Int J Mol Sci. 2022 Oct 17;23(20):12405. doi: 10.3390/ijms232012405. Int J Mol Sci. 2022. PMID: 36293262 Free PMC article.
-
Intestinal IL-33 promotes microbiota-derived trimethylamine N -oxide synthesis and drives metabolic dysfunction-associated steatotic liver disease progression by exerting dual regulation on HIF-1α.Hepatology. 2025 Jul 1;82(1):184-198. doi: 10.1097/HEP.0000000000000985. Epub 2024 Jul 10. Hepatology. 2025. PMID: 38985971 Free PMC article.
-
Ameliorative Effect of Mannuronate Oligosaccharides on Hyperuricemic Mice via Promoting Uric Acid Excretion and Modulating Gut Microbiota.Nutrients. 2023 Jan 13;15(2):417. doi: 10.3390/nu15020417. Nutrients. 2023. PMID: 36678288 Free PMC article.
-
EGCG Suppresses Adipogenesis and Promotes Browning of 3T3-L1 Cells by Inhibiting Notch1 Expression.Molecules. 2024 May 29;29(11):2555. doi: 10.3390/molecules29112555. Molecules. 2024. PMID: 38893431 Free PMC article.
References
LinkOut - more resources
Full Text Sources

