Preparation of a novel injectable in situ-gelling nanoparticle with applications in controlled protein release and cancer cell entrapment
- PMID: 35548629
- PMCID: PMC9087364
- DOI: 10.1039/c8ra06589f
Preparation of a novel injectable in situ-gelling nanoparticle with applications in controlled protein release and cancer cell entrapment
Erratum in
-
Correction: Preparation of a novel injectable in situ-gelling nanoparticle with applications in controlled protein release and cancer cell entrapment.RSC Adv. 2018 Dec 11;8(72):41376. doi: 10.1039/c8ra90099j. eCollection 2018 Dec 7. RSC Adv. 2018. PMID: 35560990 Free PMC article.
Abstract
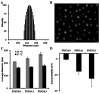
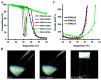
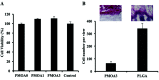
Temperature sensitive injectable hydrogels have been used as drug/protein carriers for a variety of pharmaceutical applications. Oligo(ethylene glycol) methacrylate (OEGMA) monomers with varying ethylene oxide chain lengths have been used for the synthesis of in situ forming hydrogel. In this study, a new series of thermally induced gelling hydrogel nanoparticles (PMOA hydrogel nanoparticles) was developed by copolymerization with di(ethylene glycol) methyl ether methacrylate (MEO2MA), poly(ethylene glycol) methyl ether methacrylate (300 g mol-1, OEGMA300), and acrylic acid (AAc). The effects of acrylic acid content on the physical, chemical, and biological properties of the nanoparticle-based hydrogels were investigated. Due to its high electrostatic properties, addition of AAc increases LCST as well as gelation temperature. Further, using Cy5-labelled bovine serum albumin and erythropoietin (Epo) as model drugs, studies have shown that the thermogelling hydrogels have the ability to tune the release rate of these proteins in vitro. Finally, the ability of Epo releasing hydrogels to recruit prostate cancer cells was assessed in vivo. Overall, our results support that this new series of thermally induced gelling systems can be used as protein control releasing vehicles and cancer cell traps.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Drs Zhou and Tang have a potential research conflict of interest due to a financial interest with Progenitec Inc. A management plan has been created to preserve objectivity in research in accordance with UTA policy.
Figures



Similar articles
-
A functionalized, injectable hydrogel for localized drug delivery with tunable thermosensitivity: synthesis and characterization of physical and toxicological properties.J Control Release. 2015 Jun 28;208:76-84. doi: 10.1016/j.jconrel.2015.03.003. Epub 2015 Mar 4. J Control Release. 2015. PMID: 25747144
-
Elastin based cell-laden injectable hydrogels with tunable gelation, mechanical and biodegradation properties.Biomaterials. 2014 Jul;35(21):5425-35. doi: 10.1016/j.biomaterials.2014.03.026. Epub 2014 Apr 14. Biomaterials. 2014. PMID: 24731705 Free PMC article.
-
A facile method to fabricate thermo- and pH-sensitive hydrogels with good mechanical performance based on poly(ethylene glycol) methyl ether methacrylate and acrylic acid as a potential drug carriers.J Biomater Sci Polym Ed. 2019 Oct;30(15):1375-1398. doi: 10.1080/09205063.2019.1634859. Epub 2019 Jul 3. J Biomater Sci Polym Ed. 2019. PMID: 31220422
-
Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications.Eur J Pharm Biopharm. 2014 Nov;88(3):575-85. doi: 10.1016/j.ejpb.2014.07.005. Epub 2014 Aug 1. Eur J Pharm Biopharm. 2014. PMID: 25092423 Review.
-
In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery.J Control Release. 2008 May 8;127(3):189-207. doi: 10.1016/j.jconrel.2008.01.005. Epub 2008 Jan 26. J Control Release. 2008. PMID: 18321604 Review.
Cited by
-
Recruitment of endogenous progenitor cells by erythropoietin loaded particles for in situ cartilage regeneration.Bioact Mater. 2020 Feb 6;5(1):142-152. doi: 10.1016/j.bioactmat.2020.01.007. eCollection 2020 Mar. Bioact Mater. 2020. PMID: 32072078 Free PMC article.
-
Injectable Hydrogels for Cancer Therapy over the Last Decade.Pharmaceutics. 2019 Sep 19;11(9):486. doi: 10.3390/pharmaceutics11090486. Pharmaceutics. 2019. PMID: 31546921 Free PMC article. Review.
-
Resistance Management for Cancer: Lessons from Farmers.Cancer Res. 2024 Nov 15;84(22):3715-3727. doi: 10.1158/0008-5472.CAN-23-3374. Cancer Res. 2024. PMID: 39356625 Free PMC article. Review.
References
-
- O'brien F. J. Mater. Today. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. - DOI
-
- Hou Q. Paul A. Shakesheff K. M. J. Mater. Chem. 2004;14:1915–1923. doi: 10.1039/B401791A. - DOI
-
- Yang J.-A. Yeom J. Hwang B. W. Hoffman A. S. Hahn S. K. Prog. Polym. Sci. 2014;39:1973–1986. doi: 10.1016/j.progpolymsci.2014.07.006. - DOI
-
- Choi B., Loh X. J., Tan A., Loh C. K., Ye E., Joo M. K. and Jeong B., in In Situ Gelling Polymers, Springer, 2015, pp. 5–35
LinkOut - more resources
Full Text Sources
Research Materials

