Solventless, selective and catalytic oxidation of primary, secondary and benzylic alcohols by a Merrifield resin supported molybdenum(vi) complex with H2O2 as an oxidant
- PMID: 35548632
- PMCID: PMC9086892
- DOI: 10.1039/c8ra05969a
Solventless, selective and catalytic oxidation of primary, secondary and benzylic alcohols by a Merrifield resin supported molybdenum(vi) complex with H2O2 as an oxidant
Abstract
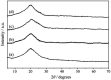
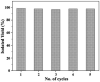
Here, we have described the synthesis, characterization and catalytic activity of a dioxo-molybdenum(vi) complex supported on functionalized Merrifield resin (MR-SB-Mo). The functionalization of Merrifield resin (MR) was achieved in two-steps viz. carbonylation (MR-C) and Schiff base formation (MR-SB). The compounds, MR-C, MR-SB and MR-SB-Mo, were characterized at each step of the synthesis by elemental, SEM, EDX, thermal, BET and different spectroscopic analysis. The catalyst, MR-SB-Mo, efficiently and selectively oxidized a wide variety of alcohols to aldehydes or ketones using 30% H2O2 as an oxidant with reasonably good TOF (660 h-1 in case of benzyl alcohol). The catalyst acted heterogeneously under solventless reaction conditions and did not lead to over oxidized products under optimized conditions. The catalyst afforded regeneration and can be reused for at least five reaction cycles without loss of efficiency and product selectivity. A reaction mechanism for the catalytic activity of MR-SB-Mo was proposed and a probable reactive intermediate species isolated.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures








References
-
- Hudlicky M., Oxidation in Organic Chemistry, ACS Monograph Series no. 186, American Chemical Society, Washington DC, 1990
- Sheldon R. A. and Kochi J. K., Metal-Catalyzed Oxidations of Organic Compounds, Academic Press, London, 1981
- Caron S. Dugger R. W. Ruggeri S. G. Ragan J. A. Ripin D. H. B. Chem. Rev. 2006;106:2943–2989. doi: 10.1021/cr040679f. - DOI - PubMed
- Beller M. Adv. Synth. Catal. 2004;346:107–108. doi: 10.1002/adsc.200404008. - DOI
- Cai J. Lu J.-Y. Chen Q.-Y. Qu L.-L. Lub Y.-Q. Gao G.-F. New J. Chem. 2017;41:3882–3886. doi: 10.1039/C7NJ00501F. - DOI
- Hao Z. Li N. Yan X. Li Y. Zong S. Liu H. Han Z. Lin J. New J. Chem. 2018;42:6968–6975. doi: 10.1039/C8NJ00329G. - DOI
- Farhadi S. Hakimib M. Maleki M. RSC Adv. 2018;8:6768–6780. doi: 10.1039/C8RA00312B. - DOI - PMC - PubMed
- de Abreu W. C. Garcia M. A. S. Nicolodi S. de Moura C. V. R. de Moura E. M. RSC Adv. 2018;8:3903–3909. doi: 10.1039/C7RA13590D. - DOI - PMC - PubMed
-
- Tornheim K. and Ruderman N. B., Intermediary Metabolism of Carbohydrate, Protein, and Fat, in Metabolic Basis of Obesity, ed. R. S. Ahima, Springer, New York, 2011, pp 25–51
-
- March J., Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, John Wiley & Sons: New York, 4 edn, 1992
-
- Kopylovich M. N., Ribeiro A. P. C., Alegria E. C. B. A., Martins N. M. R., Martins L. M. D. R. S. and Pombeiro A. J. L., Catalytic Oxidation of Alcohols: Recent Advances in Advances in Organometallic Chemistry, ed. P. J. Pérez, 2015, 63, pp 91–174
LinkOut - more resources
Full Text Sources

