Insight into trifluoromethylation - experimental electron density for Togni reagent I
- PMID: 35548651
- PMCID: PMC9087471
- DOI: 10.1039/c8ra07187j
Insight into trifluoromethylation - experimental electron density for Togni reagent I
Abstract
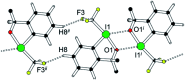
3,3-Dimethyl-1-(trifluoromethyl)-1,3-dihydro-1-λ3,2-benziodoxole represents a popular reagent for trifluoromethylation. The σ hole on the hypervalent iodine atom in this "Togni reagent" is crucial for adduct formation between the reagent and a nucleophilic substrate. The electronic situation may be probed by high resolution X-ray diffraction: the experimental charge density thus derived shows that the short intermolecular contact of 3.0 Å between the iodine and a neighbouring oxygen atom is associated with a local charge depletion on the heavy halogen in the direction of the nucleophile and visible polarization of the O valence shell towards the iodine atom. In agreement with the expectation for λ3-iodanes, the intermolecular O⋯I-Caryl halogen bond deviates significantly from linearity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Merging hypervalent iodine and sulfoximine chemistry: a new electrophilic trifluoromethylation reagent.Chem Sci. 2019 Sep 27;10(45):10516-10523. doi: 10.1039/c9sc04289j. eCollection 2019 Dec 7. Chem Sci. 2019. PMID: 32110339 Free PMC article.
-
A Tunable Trifluoromethyliodonium Reagent.Angew Chem Int Ed Engl. 2019 Jun 17;58(25):8585-8588. doi: 10.1002/anie.201903623. Epub 2019 May 17. Angew Chem Int Ed Engl. 2019. PMID: 31016799
-
Iodine(III) Reagents in Radical Chemistry.Acc Chem Res. 2017 Jul 18;50(7):1712-1724. doi: 10.1021/acs.accounts.7b00148. Epub 2017 Jun 21. Acc Chem Res. 2017. PMID: 28636313 Free PMC article.
-
Benziodoxole-based hypervalent iodine reagents for atom-transfer reactions.Chem Commun (Camb). 2011 Jan 7;47(1):102-15. doi: 10.1039/c0cc02265a. Epub 2010 Aug 27. Chem Commun (Camb). 2011. PMID: 20820531 Review.
-
Fluorofunctionalizations of C-C Multiple Bonds and C-H Bonds.Chem Pharm Bull (Tokyo). 2020;68(6):491-511. doi: 10.1248/cpb.c19-00856. Chem Pharm Bull (Tokyo). 2020. PMID: 32475852 Review.
Cited by
-
Weaving a 2D net of hydrogen and halogen bonds: cocrystal of a pyrazolium bromide with tetrafluorodiiodobenzene.Acta Crystallogr C Struct Chem. 2022 Jun 1;78(Pt 6):324-331. doi: 10.1107/S2053229622004648. Epub 2022 May 9. Acta Crystallogr C Struct Chem. 2022. PMID: 35662131 Free PMC article.
-
Exploring Orthogonality between Halogen and Hydrogen Bonding Involving Benzene.Molecules. 2021 Nov 25;26(23):7126. doi: 10.3390/molecules26237126. Molecules. 2021. PMID: 34885707 Free PMC article.
-
The Halogen Bond to Ethers - Prototypic Molecules and Experimental Electron Density.ACS Omega. 2024 Aug 5;9(32):35037-35045. doi: 10.1021/acsomega.4c05124. eCollection 2024 Aug 13. ACS Omega. 2024. PMID: 39157102 Free PMC article.
-
The many flavours of halogen bonds - message from experimental electron density and Raman spectroscopy.Acta Crystallogr C Struct Chem. 2019 Sep 1;75(Pt 9):1190-1201. doi: 10.1107/S205322961901132X. Epub 2019 Aug 22. Acta Crystallogr C Struct Chem. 2019. PMID: 31484805 Free PMC article.
-
The Relevance of Experimental Charge Density Analysis in Unraveling Noncovalent Interactions in Molecular Crystals.Molecules. 2022 Jun 8;27(12):3690. doi: 10.3390/molecules27123690. Molecules. 2022. PMID: 35744821 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

