Semi-synthesis, structural modification and biological evaluation of 5-arylbenzofuran neolignans
- PMID: 35548655
- PMCID: PMC9087020
- DOI: 10.1039/c8ra04773a
Semi-synthesis, structural modification and biological evaluation of 5-arylbenzofuran neolignans
Abstract
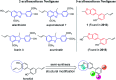
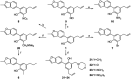
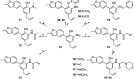
5-Arylbenzofuran neolignans, a newfound class of natural products, were reported to possess several kinds of pharmacological activities. To solve the lack of natural sources and promote the research of 5-arylbenzofuran neolignans in all fields, an available semi-synthesis methodology of 5-arylbenzofuran neolignans was developed, and a detailed structural modification was conducted. In the meantime, a one-pot process of Waker-type cyclization and Wacker-type oxidation was developed. To explore the potential of 5-arylbenzofuran neolignans as bioactive substances, 5-arylbenzofuran neolignans and their derivatives were evaluated for their cytotoxicity. As a result, a preliminary structure-activity relationship was obtained. Most derivatives revealed low cytotoxic effects suggesting that they were relatively safer than the natural 5-arylbenzofuran neolignan. Several derivatives showed high cytotoxicities which were found to be closely associated with apoptosis-inducing. The selectivity assay for cytotoxicity showed tumor cells were more sensitive to the promising compounds than normal cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Apers S. Vlietinck A. Pieters L. Phytochem. Rev. 2003;2:201–217. doi: 10.1023/B:PHYT.0000045497.90158.d2. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

