Nociception in the Glycine Receptor Deficient Mutant Mouse Spastic
- PMID: 35548669
- PMCID: PMC9082815
- DOI: 10.3389/fnmol.2022.832490
Nociception in the Glycine Receptor Deficient Mutant Mouse Spastic
Abstract
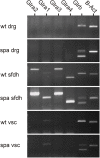
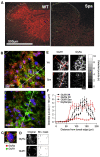
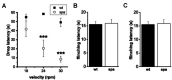
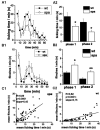
Glycine receptors (GlyRs) are the primary mediators of fast inhibitory transmission in the mammalian spinal cord, where they modulate sensory and motor signaling. Mutations in GlyR genes as well as some other genes underlie the hereditary disorder hyperekplexia, characterized by episodic muscle stiffness and exaggerated startle responses. Here, we have investigated pain-related behavior and GlyR expression in the spinal cord of the GlyR deficient mutant mouse spastic (spa). In spastic mice, the GlyR number is reduced due to a β subunit gene (Glrb) mutation resulting in aberrant splicing of GlyRβ transcripts. Via direct physical interaction with the GlyR anchoring protein gephyrin, this subunit is crucially involved in the postsynaptic clustering of heteromeric GlyRs. We show that the mutation differentially affects aspects of the pain-related behavior of homozygous Glrbspa/Glrbspa mice. While response latencies to noxious heat were unchanged, chemically induced pain-related behavior revealed a reduction of the licking time and an increase in flinching in spastic homozygotes during both phases of the formalin test. Mechanically induced nocifensive behavior was reduced in spastic mice, although hind paw inflammation (by zymosan) resulted in allodynia comparable to wild-type mice. Immunohistochemical staining of the spinal cord revealed a massive reduction of dotted GlyRα subunit immunoreactivity in both ventral and dorsal horns, suggesting a reduction of clustered receptors at synaptic sites. Transcripts for all GlyRα subunit variants, however, were not reduced throughout the dorsal horn of spastic mice. These findings suggest that the loss of functional GlyRβ subunits and hence synaptically localized GlyRs compromises sensory processing differentially, depending on stimulus modality.
Keywords: glycine receptor; glycinergic inhibition; nociception; pain; spastic; synaptic clustering.
Copyright © 2022 Groemer, Triller, Zeilhofer, Becker, Eulenburg and Becker.
Conflict of interest statement
VE works as a consultant for Disc medicine, this was, however, completely independent of the work presented here. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Becker K., Braune M., Benderska N., Buratti E., Baralle F., Villmann C., et al. (2012). A retroelement modifies pre-mRNA splicing: the murine Glrbspa allele is a splicing signal polymorphism amplified by long interspersed nuclear element insertion. J. Biol. Chem. 287, 31185–31194. 10.1074/jbc.M112.375691 - DOI - PMC - PubMed
-
- Becker L., Hartenstein B., Schenkel J., Kuhse J., Betz H., Weiher H. (2000). Transient neuromotor phenotype in transgenic spastic mice expressing low levels of glycine receptor beta-subunit: an animal model of startle disease. Eur. J. Neurosci. 12, 27–32. 10.1046/j.1460-9568.2000.00877.x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

