The intriguing biology and chemistry of fosfomycin: the only marketed phosphonate antibiotic
- PMID: 35548698
- PMCID: PMC9088020
- DOI: 10.1039/c9ra08299a
The intriguing biology and chemistry of fosfomycin: the only marketed phosphonate antibiotic
Abstract
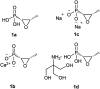
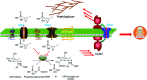
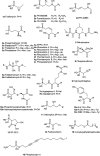
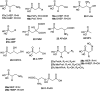
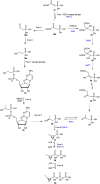
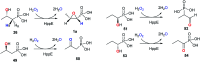
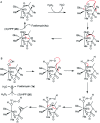
Recently infectious diseases caused by the increased emergence and rapid spread of drug-resistant bacterial isolates have been one of the main threats to global public health because of a marked surge in both morbidity and mortality. The only phosphonate antibiotic in the clinic, fosfomycin, is a small broad-spectrum molecule that effectively inhibits the initial step in peptidoglycan biosynthesis by blocking the enzyme, MurA in both Gram-positive and Gram-negative bacteria. As fosfomycin has a novel mechanism of action, low toxicity, a broad spectrum of antibacterial activity, excellent pharmacodynamic/pharmacokinetic properties, and good bioavailability, it has been approved for clinical use in the treatment of urinary tract bacterial infections in many countries for several decades. Furthermore, its potential use for difficult-to-treat bacterial infections has become promising, and fosfomycin has become an ideal candidate for the effective treatment of bacterial infections caused by multidrug-resistant isolates, especially in combination with other therapeutic drugs. Here we aim to present an overview of the biology and chemistry of fosfomycin including isolation and characterization, pharmacology, biosynthesis and chemical synthesis since its discovery in order to not only help scientists reassess the role of this exciting drug in fighting antibiotic resistance but also build the stage for discovering more novel phosphonate antibiotics in the future.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


References
-
- Freedman L. D. Doak G. Chem. Rev. 1957;57:479–523. doi: 10.1021/cr50015a003. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

