Scope and advances in the catalytic propargylic substitution reaction
- PMID: 35548716
- PMCID: PMC9085608
- DOI: 10.1039/c8ra04481c
Scope and advances in the catalytic propargylic substitution reaction
Abstract
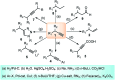
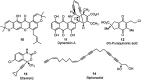
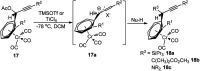
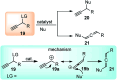
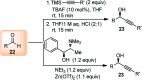
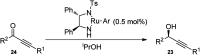
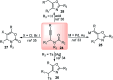
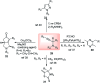
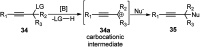
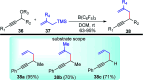
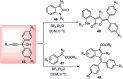
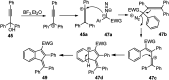
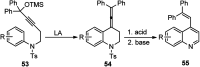
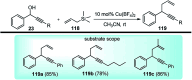
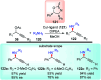
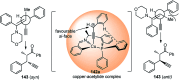
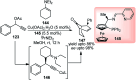
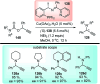
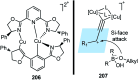
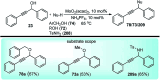
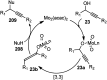
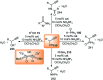
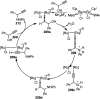
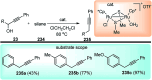
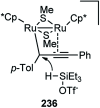
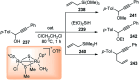
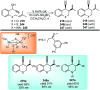
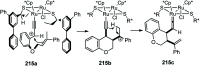
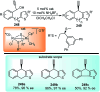
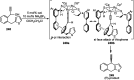
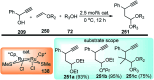
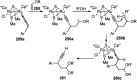
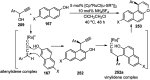
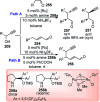
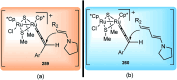
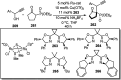
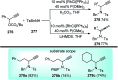
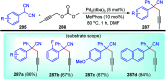
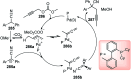
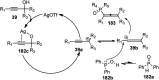
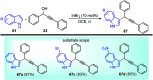
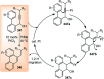
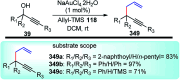
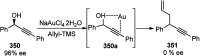
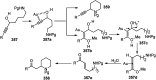
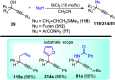
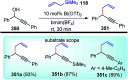
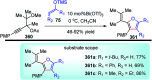
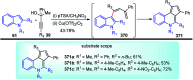
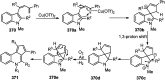
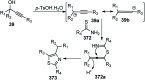
Nucleophilic displacement of the propargylic alcohol is one of the sought-after methods in the current scenario. The highly nucleophilic alkyne functional moiety along with its considerably acidic terminal hydrogen atom allows the propargylic unit to play a crucial role in organic synthesis by offering a handle for further synthetic transformations. Until 2000, the most fundamental propargylic substitution reaction was the Nicolas reaction, a multi-step transformation, developed in 1972, which involved cobalt as a stoichiometric promoter. Therefore, the direct catalytic substitution of propargylic alcohols was a highly desirable method for development. The pioneering work on the Ru-catalyzed propargylic substitution reaction in 2000 encouraged many researchers to develop several novel catalytic propargylic substitution reactions, which have made rapid progress since then. The purpose of this review is to emphasise the involvement of diverse types of Lewis acid, transition metal and Brønsted acid catalysts in the propargylic substitution reaction and provide an updated summary of the recent developments in this field. The selected examples presented here are the most significant and relevant ones and we believe that this will help the readers to comprehend the scope of the propargylic substitution reaction with diverse types of catalysts and will envisage the scientific community for the future developments in this field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
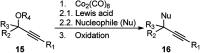

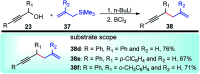
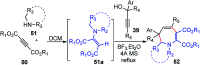

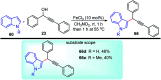
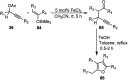
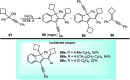
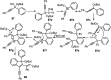
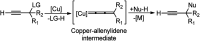
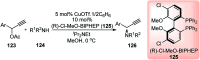
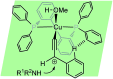
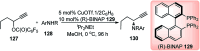
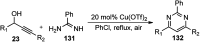
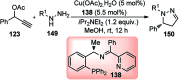
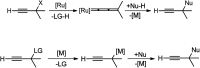
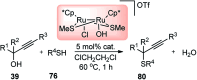
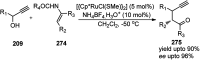

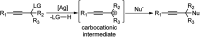
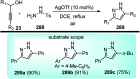
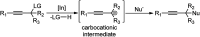
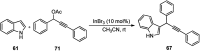
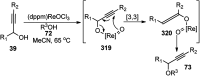
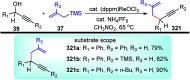
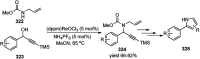
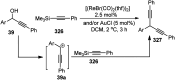


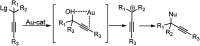



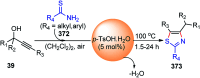
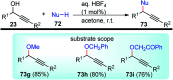
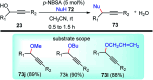
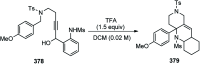
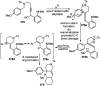
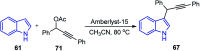
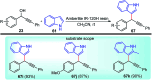
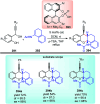
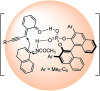


Similar articles
-
Ruthenium-catalyzed propargylic substitution reactions of propargylic alcohols with oxygen-, nitrogen-, and phosphorus-centered nucleophiles.Chemistry. 2005 Feb 18;11(5):1433-51. doi: 10.1002/chem.200400833. Chemistry. 2005. PMID: 15651018
-
Ruthenium-catalyzed propargylic substitution reaction of propargylic alcohols with thiols: a general synthetic route to propargylic sulfides.J Am Chem Soc. 2002 Dec 25;124(51):15172-3. doi: 10.1021/ja027754t. J Am Chem Soc. 2002. PMID: 12487582
-
Chiral Brønsted Acid Catalyzed Enantioconvergent Propargylic Substitution Reaction of Racemic Secondary Propargylic Alcohols with Thiols.Chemistry. 2020 Sep 1;26(49):11124-11128. doi: 10.1002/chem.202001609. Epub 2020 Jul 28. Chemistry. 2020. PMID: 32274831
-
Direct Substitution of Alcohols in Pure Water by Brønsted Acid Catalysis.Molecules. 2017 Apr 1;22(4):574. doi: 10.3390/molecules22040574. Molecules. 2017. PMID: 28368309 Free PMC article. Review.
-
Recent approaches for the synthesis of pyridines and (iso)quinolines using propargylic Alcohols.Org Biomol Chem. 2022 Aug 10;20(31):6037-6056. doi: 10.1039/d2ob00587e. Org Biomol Chem. 2022. PMID: 35678139 Review.
Cited by
-
Copper-catalyzed yne-allylic substitutions: concept and recent developments.Beilstein J Org Chem. 2024 Oct 31;20:2739-2775. doi: 10.3762/bjoc.20.232. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39498447 Free PMC article. Review.
-
Cationic indium catalysis as a powerful tool for generating α-alkyl propargyl cations for SN1 reactions.Commun Chem. 2023 Dec 16;6(1):279. doi: 10.1038/s42004-023-01048-4. Commun Chem. 2023. PMID: 38104229 Free PMC article.
-
Enantioselective construction of cyclic quaternary stereocenters via dinuclear copper catalyzed asymmetric [3 + 2] propargylation/annulation.Nat Commun. 2025 Aug 5;16(1):7191. doi: 10.1038/s41467-025-62564-6. Nat Commun. 2025. PMID: 40764491 Free PMC article.
-
Stereoselective synthesis of C3-tetrasubstituted oxindoles via copper catalyzed asymmetric propargylation.RSC Adv. 2022 Sep 21;12(41):26727-26732. doi: 10.1039/d2ra04603b. eCollection 2022 Sep 16. RSC Adv. 2022. PMID: 36320842 Free PMC article.
-
Rice husk-SiO2 supported bimetallic Fe-Ni nanoparticles: as a new, powerful magnetic nanocomposite for the aqueous reduction of nitro compounds to amines.RSC Adv. 2020 Sep 10;10(55):33389-33400. doi: 10.1039/d0ra05381c. eCollection 2020 Sep 7. RSC Adv. 2020. PMID: 35515044 Free PMC article.
References
-
- Tsuji J. Mandai T. Angew. Chem., Int. Ed. 1996;34:2589–2612. doi: 10.1002/anie.199525891. - DOI
-
- Thompson A. S. Corley E. G. Huntington M. F. Grabowski E. J. J. Tetrahedron Lett. 1995;36:8937–8940. doi: 10.1016/0040-4039(95)01955-H. - DOI
-
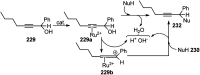
- Fusetani N. Sugano M. Matsunaga S. Hashimoto K. Tetrahedron Lett. 1987;28:4311–4312. doi: 10.1016/S0040-4039(00)96493-1. - DOI
Publication types
LinkOut - more resources
Full Text Sources

