Total synthesis of kealiiquinone: the regio-controlled strategy for accessing its 1-methyl-4-arylbenzimidazolone core
- PMID: 35548717
- PMCID: PMC9085488
- DOI: 10.1039/c8ra06676k
Total synthesis of kealiiquinone: the regio-controlled strategy for accessing its 1-methyl-4-arylbenzimidazolone core
Abstract
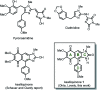
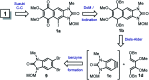
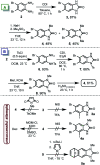
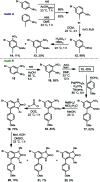
A practical, concise and straightforward total synthesis of kealiiquinone 1, a naphtho[2,3-d]imidazole alkaloid obtained from the Micronesian marine sponge Leucetta sp. was accomplished. The squaric acid chemistry to construct the 1,4-quinoid ring and the regioselective N-methylation through a benzo[c][1,2,5]selenadiazolium heterocycle are the key features in this report. The full details of the representative approaches involving the different attempted synthetic strategies are also presented. Finally a successful total synthesis of this complex secondary metabolite is described.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Protecting-Group-Free Total Synthesis and Biological Evaluation of 3-Methylkealiiquinone and Structural Analogues.J Org Chem. 2018 Sep 7;83(17):10627-10635. doi: 10.1021/acs.joc.8b01436. Epub 2018 Aug 22. J Org Chem. 2018. PMID: 30091606
-
(2E,9E)-pyronaamidine 9-(N-methylimine), a new imidazole alkaloid from the northern Mariana islands sponge Leucetta sp. cf. chagosensis.J Nat Prod. 1997 Jul;60(7):712-5. doi: 10.1021/np960673o. J Nat Prod. 1997. PMID: 9249976
-
Total syntheses and cytotoxicity of kealiiquinone, 2-deoxy-2-aminokealiiquinone and analogs.Bioorg Med Chem Lett. 2013 Nov 15;23(22):6183-7. doi: 10.1016/j.bmcl.2013.08.093. Epub 2013 Sep 6. Bioorg Med Chem Lett. 2013. PMID: 24076171 Free PMC article.
-
The emergence of the C-H functionalization strategy in medicinal chemistry and drug discovery.Chem Commun (Camb). 2021 Oct 19;57(83):10842-10866. doi: 10.1039/d1cc04083a. Chem Commun (Camb). 2021. PMID: 34596175 Review.
-
Structure and synthesis of 2-aminoimidazole alkaloids from Leucetta and Clathrina sponges.Nat Prod Rep. 2011 Mar;28(3):511-28. doi: 10.1039/c0np00001a. Epub 2010 Oct 28. Nat Prod Rep. 2011. PMID: 20981389 Review.
Cited by
-
Differential Effect of 4H-Benzo[d] [1, 3]oxazines on the Proliferation of Breast Cancer Cell Lines.Curr Med Chem. 2024;31(38):6306-6318. doi: 10.2174/0109298673292365240422104456. Curr Med Chem. 2024. PMID: 38676529
-
The Diaryliodonium(III) Salts Reaction With Free-Radicals Enables One-Pot Double Arylation of Naphthols.Front Chem. 2020 Oct 15;8:563470. doi: 10.3389/fchem.2020.563470. eCollection 2020. Front Chem. 2020. PMID: 33195052 Free PMC article.
References
-
- Carrol A. R. Bowden B. F. Coll J. C. Aust. J. Chem. 1993;46:1229–1234. doi: 10.1071/CH9931229. - DOI
-
- Akee R. K. Carrol T. R. Yoshida W. Y. Scheuer P. J. Stout T. J. Clardy J. J. Org. Chem. 1990;55:1944–1946. doi: 10.1021/jo00293a048. - DOI
-
- Kawasaki I. Taguchi N. Yamamoto T. Yamashita M. Ohta S. Tetrahedron Lett. 1995;36:8251–8254. doi: 10.1016/00404-0399(50)1770I-. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

