Synthesis and investigation of new cyclic molecules using the stilbene scaffold
- PMID: 35548740
- PMCID: PMC9085490
- DOI: 10.1039/c8ra04249g
Synthesis and investigation of new cyclic molecules using the stilbene scaffold
Abstract
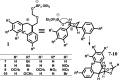
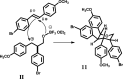
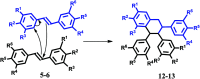
A new approach to the synthesis of asymmetrical cyclic compounds using a stilbene scaffold has been developed. The use of boron trifluoride diethyl etherate as the catalyst, both with and without paraformaldehyde, allows us to obtain new substituted dioxanes, oxanes, cyclic compounds or dimer. The analysis of products was run using experimental and theoretical methods.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
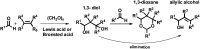
References
-
- Zhou J. Yang J. Hua B. Shao L. Zhang Z. Yu G. Chem. Commun. 2016;52:1622. doi: 10.1039/C5CC09088A. - DOI - PubMed
- Kay E. R. Leigh D. A. Zerbetto F. Angew. Chem., Int. Ed. 2007;46:72. doi: 10.1002/anie.200504313. - DOI - PubMed
- Niu Z. Gibson H. W. Chem. Rev. 2009;109:6024. doi: 10.1021/cr900002h. - DOI - PubMed
-
- Duan Q. Cao Y. Li Y. Hu X. Xiao T. Lin C. Pan Y. Wang L. J. Am. Chem. Soc. 2013;135:10542. doi: 10.1021/ja405014r. - DOI - PubMed
- Zhang H. Ma X. Nguyen K. T. Zhao Y. ACS Nano. 2013;7:7853. doi: 10.1021/nn402777x. - DOI - PubMed
- Yu G. Yu W. Mao Z. Gao C. Huang F. Small. 2015;11:919. doi: 10.1002/smll.201402236. - DOI - PubMed
-
- Harada A. Acc. Chem. Res. 2001;34:456. doi: 10.1021/ar000174l. - DOI - PubMed
- Li H. Zhang J.-N. Zhou W. Zhang H. Zhang Q. Qu D.-H. Tian H. Org. Lett. 2013;15:3070. doi: 10.1021/ol401251u. - DOI - PubMed
- Fahrenbach A. C. Bruns C. J. Li H. Trabolsi A. Coskun A. Stoddart J. F. Acc. Chem. Res. 2014;47:482. doi: 10.1021/ar400161z. - DOI - PubMed
-
- Niu Z. Huang F. Gibson H. W. J. Am. Chem. Soc. 2011;133:2836. doi: 10.1021/ja110384v. - DOI - PubMed
- Zhang Z. Luo Y. Chen J. Dong S. Yu Y. Ma Z. Huang F. Angew. Chem., Int. Ed. 2011;50:1397. doi: 10.1002/anie.201006693. - DOI - PubMed
- Tian Y.-K. Yang Z.-S. Lv X.-Q. Yao R.-S. Wang F. Chem. Commun. 2014;50:9477. doi: 10.1039/C4CC03158J. - DOI - PubMed
LinkOut - more resources
Full Text Sources

