Biosorption and bioaccumulation characteristics of cadmium by plant growth-promoting rhizobacteria
- PMID: 35548749
- PMCID: PMC9085637
- DOI: 10.1039/c8ra06270f
Biosorption and bioaccumulation characteristics of cadmium by plant growth-promoting rhizobacteria
Abstract
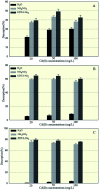
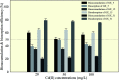
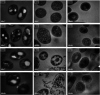
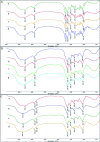
Plant growth-promoting rhizobacteria (PGPR) not only promote growth and heavy metal uptake by plants but are promising biosorbents for heavy metals remediation. However, there exist arguments over whether extracellular adsorption (biosorption) or intracellular accumulation (bioaccumulation) play dominant roles in Cd(ii) adsorption. Therefore, three cadmium-resistant PGPR, Cupriavidus necator GX_5, Sphingomonas sp. GX_15, and Curtobacterium sp. GX_31 were used to study bioaccumulation and biosorption mechanisms under different initial Cd(ii) concentrations, using batch adsorption experiments, desorption experiments, scanning electron microscopy coupled with energy dispersive X-ray (SEM-EDX) spectroscopy, transmission electron microscopy (TEM), and Fourier-transform infrared (FTIR) spectroscopy. In this study, with the increase of the initial Cd(ii) concentrations, the removal efficiency of strains decreased and the adsorption capacity improved. The highest Cd(ii) removal efficiency values were 25.05%, 53.88%, and 86.06% for GX_5, GX_15, and GX_31 with 20 mg l-1 of Cd(ii), while the maximum adsorption capacity values were 7.97, 17.13, and 26.43 mg g-1 of GX_5, GX_15, and GX_31 with 100 mg l-1 of Cd(ii). Meanwhile, the removal efficiency and adsorption capacity could be ordered as GX_31 > GX_15 > GX_5. The dominant adsorption mechanism for GX_5 was bioaccumulation (50.66-60.38%), while the dominant mechanisms for GX_15 and GX_31 were biosorptions (60.29-64.89% and 75.93-79.45%, respectively). The bioaccumulation and biosorption mechanisms were verified by SEM-EDX, TEM and FTIR spectroscopy. These investigations could provide a more comprehensive understanding of metal-bacteria sorption reactions as well as practical application in remediation of heavy metals.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict of interest and all the authors are interested to publish the manuscript.
Figures


References
LinkOut - more resources
Full Text Sources

