Efficient conversion of 5-hydroxymethylfurfural to high-value chemicals by chemo- and bio-catalysis
- PMID: 35548764
- PMCID: PMC9085621
- DOI: 10.1039/c8ra05308a
Efficient conversion of 5-hydroxymethylfurfural to high-value chemicals by chemo- and bio-catalysis
Abstract
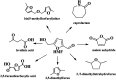
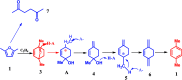
5-hydroxymethylfurfural (HMF) is a very important versatile platform compound derived from renewable biomass. The functionalized molecule with an aldehyde group, a hydroxyl group and a furan ring provides great potential for the production of a wide variety of valuable chemicals. This review highlights the latest advances in the catalytic conversion of HMF into value-added chemicals by some important reactions including (1) aerobic oxidation of HMF into furan-based aldehydes and acids such as 5-hydroxymethyl-2-furancarboxylic acid (HMFCA), 2,5-diformylfuran (DFF), and furandicarboxylic acid (FDCA), (2) reductive amination of HMF to amine, (3) the synthesis of aromatics by Diels-alder reaction followed by a dehydration reaction, (4) catalytic reduction of HMF into 2,5-bis(hydroxymethyl)furan (BHMF), and 2,5-dimethyl furan (DMF), (5) catalytic oxidation of HMF into maleic anhydride, and some other important transformations. The review mainly focuses on the recent progress in bio-catalytic, electrocatalytic, and heterogeneous catalytic transformation of HMF into high value chemicals over the past few years. Moreover, an outlook is provided to highlight opportunities and challenges related to this hot research topic.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

