Synthesis of novel genistein amino acid derivatives and investigation on their interactions with bovine serum albumin by spectroscopy and molecular docking
- PMID: 35548766
- PMCID: PMC9085648
- DOI: 10.1039/c8ra06691d
Synthesis of novel genistein amino acid derivatives and investigation on their interactions with bovine serum albumin by spectroscopy and molecular docking
Abstract
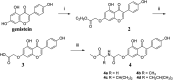
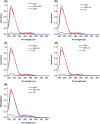
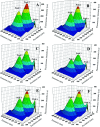
Genistein amino acid derivatives 4a-4d were synthesized and evaluated for their cytotoxic activities against MCF-7, Hela, MGC-803 and HCT-116 cell lines by MTT assays in vitro. The results revealed that compounds 4a-4d showed better activity than the parent compound genistein. Particularly, compound 4b displayed the most significant anticancer activity against MGC-803 with an IC50 value of 12.08 μM. In addition, the mechanisms of interaction between genistein, compounds 4a-4d and BSA were investigated via multi-spectroscopic techniques such as ultraviolet (UV) spectroscopy, fluorescence, circular dichroism (CD), and molecular docking under physiological conditions. The results suggested that endogenous fluorescence of BSA could be quenched by genistein and compounds 4a-4dvia forming BSA-compound complex, which meant a static quenching mechanism was involved. The negative values of enthalpy (ΔH) and entropy (ΔS) indicated that interactions between BSA and the ligands were spontaneous, and hydrogen bonding and van der Waals interactions were involved in the BSA-compound complexion formation. The UV, synchronous and 3D fluorescence results revealed that the micro-environment of tryptophan and conformation of BSA were changed after binding to ligands. CD analysis demonstrated the variation in the secondary structure and that the α-helix content of BSA decreased. Eventually, molecular docking was executed to forecast the binding forces and binding sites between BSA and compounds 4a-4d.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Carter D. C. Chang B. Ho J. X. Keeling K. Krishnasami Z. FEBS J. 1994;226:1049–1052. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

