Characterization and osteogenic evaluation of mesoporous magnesium-calcium silicate/polycaprolactone/polybutylene succinate composite scaffolds fabricated by rapid prototyping
- PMID: 35548789
- PMCID: PMC9086718
- DOI: 10.1039/c8ra06281a
Characterization and osteogenic evaluation of mesoporous magnesium-calcium silicate/polycaprolactone/polybutylene succinate composite scaffolds fabricated by rapid prototyping
Abstract
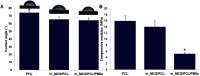
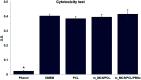
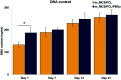
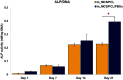
The properties of scaffolds for bone tissue engineering, including their biocompatibility, highly interconnected porosity, and mechanical integrity, are critical for promoting cell adhesion, proliferation, and osteoinduction. We used various physical and biological assays to obtain in vitro confirmation that the proposed composite scaffolds are potentially suitable for applications to bone tissue engineering. The proposed new composite scaffolds, which we fabricated by a rapid prototyping technique, were composed of mesoporous magnesium-calcium silicate (m_MCS), polycaprolactone (PCL), and polybutylene succinate (PBSu). We systematically evaluated the characteristics of the composite scaffolds, such as the hydrophilicity and bioactivity. We also investigated the proliferation and osteogenic differentiation of human mesenchymal stem cells (MSCs) scaffolded on the m_MCS/PCL/PBSu composite. Our results showed that, compared to the m_MCS/PCL scaffold, the m_MCS/PCL/PBSu scaffold has improved water absorption, in vitro degradability, biocompatibility, and bioactivity in simulated body fluid, while its mechanical strength is reduced. Moreover, the results of the cytotoxicity tests specified in ISO 10993-12 and ISO 10993-5 clearly indicate that the m_MCS/PCL scaffold is not toxic to cells. In addition, we obtained significant increases in initial cell attachment and improvements to the osteogenic MSC differentiation by replacing the m_MCS/PCL scaffold with the m_MCS/PCL/PBSu scaffold. Our results indicate that the m_MCS/PCL/PBSu scaffold achieves enhanced bioactivity, degradability, cytocompatibility, and osteogenesis. As such, this scaffold is a potentially promising candidate for use in stem cell-based bone tissue engineering.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures






Similar articles
-
Enhanced biocompatibility and osteogenic potential of mesoporous magnesium silicate/polycaprolactone/wheat protein composite scaffolds.Int J Nanomedicine. 2018 Feb 26;13:1107-1117. doi: 10.2147/IJN.S157921. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29520139 Free PMC article.
-
Biodegradable mesoporous calcium-magnesium silicate-polybutylene succinate scaffolds for osseous tissue engineering.Int J Nanomedicine. 2015 Oct 28;10:6699-708. doi: 10.2147/IJN.S92598. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26604746 Free PMC article.
-
Effects of magnesium silicate on the mechanical properties, biocompatibility, bioactivity, degradability, and osteogenesis of poly(butylene succinate)-based composite scaffolds for bone repair.J Mater Chem B. 2016 Dec 28;4(48):7974-7988. doi: 10.1039/c6tb02429g. Epub 2016 Nov 25. J Mater Chem B. 2016. PMID: 32263787
-
Influences of mesoporous magnesium calcium silicate on mineralization, degradability, cell responses, curcumin release from macro-mesoporous scaffolds of gliadin based biocomposites.Sci Rep. 2018 Jan 9;8(1):174. doi: 10.1038/s41598-017-18660-9. Sci Rep. 2018. PMID: 29317753 Free PMC article.
-
Fabrication and Biological Activity of 3D-Printed Polycaprolactone/Magnesium Porous Scaffolds for Critical Size Bone Defect Repair.ACS Biomater Sci Eng. 2020 Sep 14;6(9):5120-5131. doi: 10.1021/acsbiomaterials.9b01911. Epub 2020 Aug 3. ACS Biomater Sci Eng. 2020. PMID: 33455263
Cited by
-
Fabrication of Rosuvastatin-Incorporated Polycaprolactone -Gelatin Scaffold for Bone Repair: A Preliminary In Vitro Study.Cell J. 2024 Jan 31;26(1):70-80. doi: 10.22074/cellj.2023.2009047.1391. Cell J. 2024. PMID: 38351731 Free PMC article.
-
Thermomechanical Properties of SiC-Filled Polybutylene Succinate Composite Fabricated via Melt Extrusion.Polymers (Basel). 2020 Feb 11;12(2):418. doi: 10.3390/polym12020418. Polymers (Basel). 2020. PMID: 32054110 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

